- Home
- Companies
- north america
- introducer sheath
Refine by
Introducer Sheath Suppliers In North America
17 companies found

based inNewark, DELAWARE (USA)
Founded in 1958, Gore is a global materials science company with more than 13,000 Associates spanning five continents and thousands of products across industries — from high-performance fabrics to implantable medical devices and products that reduce ...
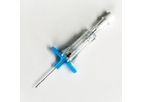
Physicians performing TIPS procedures request accessories that provide instant feedback. The GORE TIPS Set, composed of the GORE TIPS Needle and GORE TIPS Sheath, provides this ...

based inAlpharetta, GEORGIA (US) (USA)
Founded by Fred L. Aycock in 1992, Remington Medical Inc. specializes in medical devices. The maintenance, time, and money put into cleaning and sterilizing medical devices led us to develop some of the first disposable cables, some still in use ...
Introducer set used for placement of a .038-inch diameter guide wire into the vascular ...

based inSouth Jordan, UTAH (USA)
Founded in 1987, Merit Medical Systems, Inc. is a leading manufacturer and marketer of proprietary disposable medical devices used in interventional, diagnostic and therapeutic procedures, particularly in cardiology, radiology, oncology, critical ...
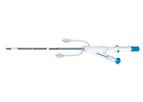
The Prelude ACT (Activated Clotting Time) sheaths are part of Merit’s comprehensive family of vascular access products. These half-size sheaths allow easy blood draw through sheath side arm with or without catheters in place. The ACT ...

based inSparks, NEVADA (USA)
Neo Medical, Inc. is an innovator in vascular access medical devices. Noted for its high value the Neo-Magic line of vascular access devices and accessories designed specifically for neonatal and pediatric patients. Neo Medical services specialize ...
1.9/2.0 Fr, 27ga Sharps Safety Needle & Tearaway Sheath Introducer. Small profile creates ultra smooth sheath to needle transition and features funnel lead-in and ...

based inScottsdale, ARIZONA (USA)
Innovative Health is an advanced, FDA regulated reprocessing company focused on electrophysiology devices. This means we gain FDA clearance to reprocess and sell back FDA single-use cardiology devices that have been used one or more times. ...
Steerable introducer sheaths can be very expensive and they are a challenge from a reprocessing perspective, since faulty sheaths can be very dangerous for the patient. To reprocess them properly, ...

based inCarlsbad, CALIFORNIA (USA)
CeloNova BioSciences, Inc. is developer and manufacturer of a portfolio of novel surfaced coated technologies, including the proprietary Polyzene-F nanocoating. This next-generation nanocoating is the result of years of rigorous scientific research ...

based inSomerset, NEW JERSEY (USA)
Terumo Interventional Systems (TIS), a division of Terumo Medical Corporation, is a market leader in minimally invasive entry site management, lesion access, and interventional technologies. At Terumo Interventional Systems (TIS), we believe that ...
The GLIDESHEATH Introducer System is a radial access kit specifically designed and intended for smooth atraumatic radial access to minimize the risk of scarring and ...

based inCarlsbad, CALIFORNIA (USA)
Acutus Medical, Inc. was founded in 2011 and is based in Carlsbad, CA. Our products are co-developed in the U.S. and Europe utilizing the skills of an exceptional team of medical scientists, biomedical engineers, and other professionals. Acutus ...
Stable and Reliable Reference. Unipolar Pacing and Recording for VT ...

based inReutlingen, GERMANY
It is probably a combination of many factors that has made us successful since 2004: the good collaboration of a medium-sized family company; the site in the heart of Germany’s medical technology region around the University of Tübingen; our ...
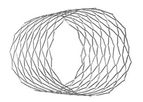
The semi-flexible hybrid cell Cobalt Chromium bare stent has been specifically designed for the effective treatment of Coarctation. Available in XL and ...

based inChampionsGate, FLORIDA (USA)
Our purpose is to improve health of the patients all around the world with our strong, hardworking, dedicated and clinically driven biomedical solutions team. Our products and technologies are mostly focused on endovascular treatment solutions with ...

based inIrvine, CALIFORNIA (USA)
JenaValve is a clinical-stage medical device company developing the first transcatheter heart valve technology that is uniquely designed for the minimally invasive treatment of both severe aortic regurgitation and stenosis. JenaValve is passionate ...
The JenaValve Transfemoral Delivery Catheter is designed to safely treat patients while delivering consistent, reproducible performance for physicians. The system features the ...

based inCaesarea Industrial Park (N), ISRAEL
V-WAVE was established in Israel in 2009 with a focus on developing percutaneous implantable technologies for patients with chronic heart failure. Technology designed by V-Wave, highlighted by its Ventura Interatrial Shunt System, was designed to ...
The V-Wave Ventura Interatrial Shunt is a novel, hourglass-shaped implantable device. The shunt is designed to enable shunting of blood across the interatrial ...

based inBoerne, TEXAS (USA)
Prytime Medical Devices, Inc., an innovative medical device company, designs, develops and commercializes minimally invasive solutions for hemorrhage control. The company’s flagship product is the ER-REBOA™ Catheter, a 7 Fr compatible balloon ...
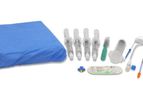
The REBOA Convenience Set is compatible with all Prytime Medical™ REBOA catheters and provides the clinician with the components needed for common femoral artery access, REBOA placement, and external fixation of the ...

based inOakville, ONTARIO (CANADA)
CHS Ltd. (est. 1967) is a privately held medical device manufacturer and specialty distributor located in Oakville, Ontario. Customers served are in the acute hospital and non-acute healthcare space in Canada and Internationally. CHS ...

based inMiami Lakes, FLORIDA (USA)
Cordis is an independent, customer-focused provider of interventional cardiovascular technologies. During our 60-year history we’ve established a legacy of pioneering breakthrough technologies, including the first guiding catheters and coronary drug ...
The EXOSEAL VCD is indicated for femoral artery puncture site closure, reducing times to hemostasis and ambulation in patients who have undergone diagnostic or interventional catheterization procedures using a standard 5F, 6F, or 7F vascular ...

based inAbbott Park, ILLINOIS (USA)
We create breakthrough products – in diagnostics, medical devices, nutrition and branded generic pharmaceuticals – that help you, your family and your community lead healthier lives, full of unlimited possibilities. Today, 109,000 of us are working ...
Precision. Performance. Results. Excellent precision, flexibility and proven clinical results in the iliacs. Precision; Triaxial technology designed to absorb stored energy and minimize friction during deployment to ensure precise stent ...

based inMontreal, QUEBEC (CANADA)
QXMedical was founded in 2007 in Montreal, Canada on the principle of providing uncompromised value and quality in underserved high-growth segments of the medical device market. Today, the company is known for its innovative solutions for complex, ...
The Q50X Stent Graft Balloon Catheter offers ULTRA low 10 Fr sheath compatibility with the broadest balloon diameter for use with aortic stent grafts that treat abdominal and thoracic aneurysms (AAA/TAA). The catheter offers exceptional inflation ...