- Home
- Companies
- australasia
- thrombectomy
Show results for
Refine by
Thrombectomy Suppliers Serving Australasia
14 companies found

based inSan Mateo, CALIFORNIA (USA)
For 20+ years, most believed we had only a few hours after a stroke to provide treatment—but we were never satisfied with that thinking. We theorized that stroke evolution was highly variable, the conventional short treatment windows could be ...
Rapid CTP is the only clinically validated software with an FDA indication to aid in the selection of patients for acute stroke ...

based inEl Paso, TEXAS (USA)
Seisa Medical is a global full-service contract manufacturer of Class II and Class III medical devices and specialty components, ranging from implantable stents and tubing sets to pediatric and orthopedic-care products. We serve customers through ...

based inAlameda, CALIFORNIA (USA)
Penumbra, Inc., headquartered in Alameda, California, is a global healthcare company focused on innovative therapies. Penumbra designs, develops, manufactures and markets novel products and has a broad portfolio that addresses challenging medical ...
The Penumbra JET™ D Reperfusion Catheter is intended for use in the revascularization of patients with acute ischemic stroke secondary to large vessel occlusion. The Penumbra JET D is designed to navigate smaller, distal vessels and extract ...

based inOxford, UNITED KINGDOM
We specialize in the creation of AI-powered imaging biomarkers to enable precision medicine for better treatment decisions. Since launching as a spin-out from the University of Oxford, we have developed award-winning, AI-powered imaging biomarkers ...
e-Stroke is a CE-marked collection of tools that use our state-of-the-art AI algorithms to support doctors by providing real-time interpretation of brain scans to help guide treatment and transfer decisions for stroke patients, allowing more ...

based inCorona, CALIFORNIA (USA)
For over 20 years, United Endoscopy has been a leader in providing state-of-the-art refurbished medical equipment and advanced equipment repair to its customers worldwide. Since its founding in 1996 by Jeremy Cooper, United Endoscopy has ...

based inEden Prairie, MINNESOTA (USA)
Surmodics’ leadership in surface technologies dates to our development of the drug-delivery coating for the first drug-eluting stent. Since then, we’ve consistently pioneered breakthrough lubricious, hemocompatible, and drug-delivery COATINGS for ...
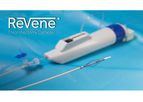
The Vetex Medical ReVene® Thrombectomy Catheter has been engineered with a patented dynamic cage technology that enables the predictable removal of wall adherent thrombus in a single session without the need for ...
based inZhuhai, CHINA
NeuroSafe Medical Co., Ltd. specializes in the development of state-of-the-art medical technologies designed to improve patient outcomes and advance modern healthcare solutions. With a commitment to ensuring safety and efficacy, the company focuses ...
The DredgerTM Stent Retriever is a specialized medical device designed to rapidly address ischemic strokes by effectively removing thrombotic blockages in cerebral arteries. This device is crafted for use within eight hours of symptom onset, making ...

based inIrvine, CALIFORNIA (USA)
Walk Vascular was founded in 2010, with a mission to treat and transform the lives of patients suffering from peripheral arterial and venous disease. Its JETi Thrombectomy System (JETi) is manufactured within The Company's 19,000 sq. ft., Irvine, ...
The JETi AIO Peripheral Thrombectomy System is intended to remove/aspirate fluid and break-up soft emboli and thrombus from the peripheral vasculature and to sub selectively infuse/deliver diagnostics or therapeutics with or without ...

based inMinneapolis, MINNESOTA (USA)
At Medtronic, we believe in the power of medical technology to improve lives. Seven decades ago, our co-founder invented the battery-powered pacemaker. Today, we are among the largest medical device companies in the world. With operations in 150 ...
The React™ 68 catheter and the React™ 71 catheter feature a coil and braid design along with end-to-end nitinol construction — easing navigation to the M1 and M2 segments. Combined with the Riptide™ aspiration system, these ...

based inBrunswick, AUSTRALIA
Avania is an integrated global, full-service CRO with specialized expertise in medical device, novel technology, and combination products. They advance products from feasibility all the way through post-approval trials in analytics, clinical trials, ...

based inChambly, FRANCE
ISOMed is specialized in disposable medical devices for vascular access. Since 1990, we propose therapeutic solutions in vascular surgery, oncology and digestive surgery. Our products meet the technical requirements practitioners demand everywhere ...
ISOMed Thrombectomy Catheters - single lumen are made with X.R.O. polyurethane. They are colour coded for easy identification. The specially designed latex balloon, fixed with two sutures, is perfectly symmetrical. ...

based inRouen, FRANCE
Founded in 2009 by Philippe Bencteux, MD, Robocath designs, develops and commercializes robotic solutions to treat cardiovascular diseases. As an active player in the evolving medical robotic industry, these innovative solutions aim to make medical ...

based inMississauga, ONTARIO (CANADA)
Baylis Medical Technologies is a company focused on developing and commercializing advanced medical devices used in endovascular therapy and other vascular procedures. Their products, such as the PowerWire Pro RF Guidewire, are designed to help ...
The PowerWire® Pro RF Guidewire is designed for precision and control in complex vascular procedures, aiding the introduction of diagnostic and therapeutic devices into various parts of the human vasculature, such as intracardiac, renal, and ...

based inStenløse, DENMARK
Mermaid Medical Group is a privately owned company established in 2007. With our Nordic roots and headquarters in Denmark, we value cooperation, helpfulness, and openness. We develop, manufacture, and distribute medical devices to hospitals and end ...
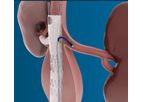
The D*Clot HD is a fully disposable system that is ready to use. No assembly required. It is indicated for mechanical declotting of AV dialysis fistulae and ...