- Home
- Companies
- canada nova scotia
- medical device regulation
Refine by
Industries served
- Medical / Health Care
- Monitoring and Testing
- Chemical & Pharmaceuticals
- Environmental
- Health and Safety
- Manufacturing, Other
- Waste and Recycling
- Aerospace & Air Transport
- Oil, Gas & Refineries
- Soil and Groundwater
- University / Academia / Research
- Food and Beverage
- Energy
- Construction & Construction Materials
- Banking & Finance / Insurance / Legal
- Glassware
- Retail
- Packaging
- Plastics & Resins
- Water and Wastewater
- Agriculture
- Air and Climate
Medical Device Regulation Suppliers Serving Canada Nova Scotia
121 companies found

Premium
based inHorsholm, DENMARK
At TannerMedico A/S, our aim is to help you sleep comfortable, breathe easier and live healthier. We truly believe that any good treatment aims at killing the cause and not just the symptoms. We are a Scandinavian company with the corporate ...
3 bottles of Asonor anti snoring solution, covering 3 month peaceful night’s sleep for you and your partner. Plus a travel kit. Award winning and Clinically proven Asonor anti snoring solution is ranked as one of the world’s best ...

based inSanta Clara, CALIFORNIA (USA)
Since 1992, Omnicell has been committed to transforming the pharmacy care delivery model to dramatically improve outcomes and lower costs. Through the vision of the autonomous pharmacy, a combination of automation, intelligence, and ...

based inSchönenbuch, SWITZERLAND
BÜHLMANN is a fully independent, medium sized and family owned Swiss company. The company was founded in 1976 and has continuously developed over the years. BUHLMANN continues to renew and enlarge its product port and in combining innovation with ...

based inElst, NETHERLANDS
Artinis Medical Systems specializes in non-invasive NIRS devices for brain and muscle imaging, including applications for children and infants. Their products are geared towards research rather than clinical diagnostics, aligning with the Medical ...
The OctaMon Mini by Artinis is a wearable, 8-channel functional near-infrared spectroscopy (fNIRS) device tailored for pediatric research. It is designed to facilitate the monitoring of brain activity during everyday activities, ...

based inMiami, FLORIDA (USA)
Bio-Tissue, Inc. was founded in 1997 to help address unmet needs for improved surgical and therapeutic alternative to treat ocular surface conditions; one that would serve as a non-immunogenic tissue replacement and would help facilitate tissue ...

based inWenzhou, CHINA
Global player specialized in manufacture and distributor of medical products, with a presence in 36 countries. A group with five (5) subsidiary factories and eight (8) joint venture manufacture base. A product scope more than 110 products, with a ...

based inWarsaw, POLAND
Corning HTL SA. is one of the world’s major manufacturers of liquid handling equipment. Over 35 years of experience in design and production of manual dosing systems, together with our strong focus on customer satisfaction allowed us to gain the ...

based inAnaheim, CALIFORNIA (USA)
Sechrist Industries has developed and manufactured hyperbaric chambers since 1973. Using our state-of-the-art facility, we design and ship to over 172 countries. We make sure to monitor and track every step of the hyperbaric chamber manufacturing ...

based inTourcoing, FRANCE
Launched in 2008, Spring Thread quickly became the reference thread for physicians who are looking for effective traction for their patients, a long lasting effect, and an easy to install product with features that ensure all the necessary security ...

based inAnkara, TURKEY
Sümer A.S. was established in 1981 in Ankara to proved services in the medical device sector. It has aimed advancement since the day of its establishment by also taking growth and compliance with the contemporary technologies and protecting the ...

based inSurrey, UNITED KINGDOM
Surtex Instruments is a medical instruments manufacturer from United Kingdom. Our commitment to innovative solutions, excellence and quality craftsmanship in the manufacture surgical instruments has made us one of the most trusted brands by ...

based inAustin, TEXAS (USA)
Emergo by UL is a leading regulatory consulting firm specializing in global medical device and IVD compliance. Our comprehensive solution is designed to help you achieve and maintain regulatory and commercial success. With a presence on six ...
It is easy to make mistakes in the regulatory process that can delay market entry or incur unnecessary costs. With help from Emergo’s medical device RA/QA consultants, you can pursue the most efficient regulatory route to approval and ...

based inCleveland, OHIO (USA)
Gebauer Company specializes in producing topical anesthetic products, with over a century of experience in the field. Known for its dedication to enhancing patient comfort, Gebauer's products are utilized in healthcare environments to improve ...
Gebauer's Spray and Stretch is an instant topical anesthetic skin refrigerant that effectively manages myofascial pain and trigger point release when used in conjunction with the spray and stretch technique. Our spray is also indicated for treating ...

based inJena, GERMANY
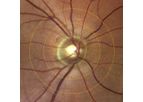
Imedos is the leading expert in microcirculation and microvascular health of the retina. For more than 25 years, we have been developing and distributing innovative systems for research in the field of ...
The static vessel analysis offers an innovative solution for the evaluation of the state of blood vessels. It is designed for use in research and provides important parameters and information about the risk of subjects for cardiovascular diseases ...

based inManchester, UNITED KINGDOM
Globus Group is the UK’s largest PPE manufacturer and a leading European provider of innovative protection technology solutions. With over 25 years’ heritage Globus is leading the way in manufacturing resilience and sustainability. The company is ...

based inHangzhou, CHINA
Chemical Inspection and Regulation Service (CIRS) is a leading product safety and chemical management consulting firm providing valued product regulatory compliance service, tailored solutions and original information to help our clients gain ...
Medical Devices and In Vitro Diagnostics Product Regulatory Compliance in China. Pre-market Investigation & Analysis. Medical Devices Registration & Approval. Clinical Trial Consulting. Manufacturing and Distributing License Approval. ...

based inDelta, BRITISH COLUMBIA (CANADA)
Innovatek Medical Inc. is a manufacturer, importer and distributor of medical devices, founded in 1990. We're a Canadian company providing rapid diagnostic kits in the areas of women's health, drugs of abuse, and infectious diseases. We also supply ...

based inKanpur, INDIA
Set up in 2003 exclusively for the international market, Aditya Dispomed Products Pvt. Ltd. produces a wide range of surgical blades, Disposable Scalpels and Safety Scalpels under the Kiato brand name. Our blades command respect from the finest ...

based inSheffield, UNITED KINGDOM
Founded in Sheffield during 1932, Swann-Morton have become a world leader in the manufacture of surgical blades, scalpels and handles. It is a name respected globally for quality, precision, consistency and reliability and recognised for combining ...

based inShirley, CALIFORNIA (USA)
STEMart is an industry-leading eCommerce platform, with an expanded global footprint, we now have a broad portfolio of more than 10,000 products. In the fields of science, technology, and engineering, from the discovery stage towards the ...
CE marking is the manufacturer's declaration that their product complies technically and administratively with the essential regulatory requirements of Medical Device Directive 93/42/EEC (MDD) and its supplementary Directive 2007/47/EEC, Active ...