Refine by
Diagnostic Device Development Suppliers Serving Eritrea
119 companies found

based inSan Francisco, CALIFORNIA (USA)
Tempo Bioscience, Inc (Tempo) develops human stem cells (human iPSC technology) based cell models and biosensor enabling technologies for preclinical drug discovery & development, biobanking, in vitro diagnostics, and biomarker development. Our ...
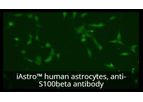
Astrocytes, also known as Astroglia, belong to the family of glial cells in the human central and peripheral nervous system. They have been shown to have central roles in the maintenance of neuronal metabolism and neurotransmitter synthesis. They ...

based inCoralville, IOWA (USA)
A champion of life sciences research for more than 30 years, IDT develops and manufactures nucleic acid products that support the life sciences industry. Areas of focus include academic and commercial research, agriculture, medical diagnostics, ...

based inSan Diego, CALIFORNIA (USA)
Quidel Corporation (Nasdaq: QDEL) is a California-based leading diagnostic healthcare manufacturer serving to enhance the health and well-being of people around the globe through the development of diagnostic solutions that can lead to improved ...

based inSalem, NEW HAMPSHIRE (USA)
We've manufactured over 8 million devices, three billion sensors, and developed the first FDA-cleared blood glucose meter/app for iPhone®. We've been featured by the New York Times and Fast Company, and work with some of the world's largest ...

based inMadrid, SPAIN
Biotools B&M Labs, S.A. is a Spanish biotechnology company with a global presence, created with the mission of combining scientific excellence with the development of quality solutions for its customers in the Life Sciences, Biomedical and ...

based inWijchen, NETHERLANDS
Future Diagnostics is the obvious choice for in vitro diagnostic (IVD) assay development – for start-ups, mid-sized biotech companies and multinationals in the global IVD medical device market. With 20+ years of expert experience and 150 IVD ...

based inLund, SWEDEN
AcouSort is an innovative technology company with high focus on products and solutions for automated preparation of biological samples for researchers and life science companies. The core technology is acoustofluidics where a combination of ...

based inFairfield, AUSTRALIA
Axxin provides platforms and diagnostic products for biomedical applications that bridge the gap from research to market with world leading innovation, capability and cost effectiveness. The company's goal is to take the cost and complexity out of ...

based inWoodland Hills, CALIFORNIA (USA)
EntroGen offers end-to-end molecular diagnostic solutions by providing hardware, reagents and software. Starting from automated nucleic acid extraction and culminating in genomic interpretation with proprietary software embedded for key decision ...

based inSharnbrook, UNITED KINGDOM
AgPlus Diagnostics is proud to present the Agilis Reader. A portable diagnostic solution, comparable to any fixed laboratory analyzer, The Agilis Reader is a quantitative diagnostic reader that can be used in multiple market applications that when ...

based inKAARINA, FINLAND
Labmaster Oy Ltd. is a company engaged in the design, development, and manufacturing of various products, ensuring they meet specified criteria through proper storage, usage, and transportation guidelines. They emphasize adherence to instructions ...

based inHamburg, GERMANY
altona Diagnostics is a medical diagnostic company that develops and manufactures in vitro diagnostic tests for the PCR based detection of pathogens such as viruses, bacteria or parasites. Headquartered in Hamburg-Altona, Germany, altona Diagnostics ...

based inMonmouth Junction, NEW JERSEY (USA)
DiamiR is a privately held molecular diagnostics company focused on developing minimally invasive tests for detection and monitoring of pathology based on quantitative analysis of organ-enriched microRNA signatures in plasma for screening, patient ...

based inDurham,, NORTH CAROLINA (USA)
Founded in 1998, Cancer Diagnostics, Inc. (CDI) is a leading developer and manufacturer of histology, cytology, surgical pathology and immunohistochemistry diagnostic supplies. CDI developed the first commercially available 7-dye color kit for ...

based inLincolnshire, ILLINOIS (USA)
Sysmex America, Inc. distributes and supports automated in vitro diagnostic hematology, flow cytometry, technology solutions, coagulation and urinalysis analyzers, reagents and information systems for laboratories and healthcare facilities in North ...

based inBelfast, UNITED KINGDOM
At Diaceutics we believe that every patient should have access to the right treatment at the right time. We provide the world’s leading pharmaceutical companies with an end-to-end solution for the launch of precision medicine diagnostics enabled by ...

based inSuwon-si, SOUTH KOREA
SD BIOSENSOR is a professional in-vitro diagnostics company with aims of contributing to higher life quality by diagnosing illnesses accurately and quickly. SD BIOSENSOR is researching and developing quick diagnostics, immunodiagnostics, molecular ...

based inHuesca, SPAIN
Vitassay develops and distributes reliable and certified solutions for the early detection of different pathogens which cause infectious diseases in humans. Our greatest asset is to offer a complete service for every customer to exceed their ...

based inSan Antonio, TEXAS (USA)
SA Scientific, Ltd. has proudly served clinical laboratories worldwide since 1984. We design, develop and manufacture a wide range of One-Step rapid tests for fertility and infectious diseases. SA Scientific has a tradition of producing excellent ...

based inBuffalo, NEW YORK (USA)
At ZeptoMetrix, we’ve always understood that research success requires a team approach. That is why our mission to be “a partner to our customers” remains the focal point as we, together with our industrial partners, endeavor to change the global ...
The Zika Antibody Control Pack consists of onevial each ofIgG, IgM and negative antibody controls. The IgG and IgM controls contain anti-Zika NS1 mouse monoclonal antibody conjugated to purified human IgG or IgM, respectively. All controls are ...