Refine by
Industries served
Software For Medical Devices Suppliers Serving France
32 companies found

based inAtlanta, GEORGIA (US) (USA)
Sequenex is at the forefront of medtech software development, providing expertise in Software as a Medical Device (SaMD) and digital health solutions. Their offerings include prebuilt platforms like NEX that allow rapid deployment and significant ...

based inJena, GERMANY
Imedos is the leading expert in microcirculation and microvascular health of the retina. For more than 25 years, we have been developing and distributing innovative systems for research in the field of ...

based inFramingham, MASSACHUSETTS (USA)
At Definitive Healthcare, our passion is to transform data, analytics and expertise into healthcare commercial intelligence. We help clients uncover the right markets, opportunities and people, so they can shape tomorrow’s healthcare industry. Our ...

based inDublin, IRELAND
At S3 Connected Health we create digital health solutions and connected medical devices that improve the lives of people with acute and chronic conditions. Our 200-strong team throughout Europe and the US have delivered award-winning solutions for ...
These solutions allow pharma to holistically tackle disease categories and improve healthcare outcomes. We take our clients from concept, through creation, development of software as a medical ...
based inShanghai, CHINA
LinaTech, headquartered in Sunnyvale, California, is a leading manufacturer of medical devices and software for treating cancer with radiotherapy. The company supplies informatics software for managing comprehensive cancer clinics, radiotherapy ...

based inChelmsford, MASSACHUSETTS (USA)
ZOLL, an Asahi Kasei company, develops and markets medical devices and software solutions that help advance emergency care and save lives, while increasing clinical and operational efficiencies. With products for defibrillation and cardiac ...

based inIndianapolis, INDIANA (USA)
Drug Free Therapeutix (DFTx) provides intelligent software for medical device companies in or entering the lucrative $4BB pain management device market. Our platform technology, known as Extraneural Activation Control Technology (EXACT), fine-tunes ...

based inLanghorne, PENNSYLVANIA (USA)
Syncro Medical takes quality very seriously. We’re software craftsmen – attention to quality is part of our culture and embedded in every aspect of how we deliver our services. Competition within the medical device industry continues to intensify. ...
In many cases, the Requirements phase is the most difficult stage of new product development. Progress is stymied as companies struggle to define the requirements for an innovative new product. MIT’s Sloane Management Review refers to the ...

based inElgin, TEXAS (USA)
Northgate Technologies, Inc. is a worldwide leader in the design and production of minimally invasive software controlled medical devices and specialty products for stone management and fluid management in surgical environments. We offer a growing ...

based inDarmstadt, GERMANY
The development and support of our software solutions is carried out exclusively in Germany by qualified BAYOOMED employees. This allows us to personally accompany and advise you on your project right from the start. You tell us your vision and we ...

based inUlm, GERMANY
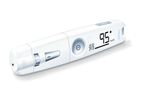
Beurer - Specialist for health & well-being. We offer a range of more than 500 products, and ever since 1919 we have been delivering what our claim promises: health and wellbeing. Our product ranges will ensure you feel great all-round. From medical ...
All-in-one the blood glucose monitor combines a measuring device, plug-in USB/onboard software and lancing device in one. The simple measurement marking and the blood volume check are just two of the outstanding features of the 3-in-1 compact ...

based inValkenswaard, NETHERLANDS
Established in 2004, 2M Engineering is an esteemed privately-owned SME enterprise renowned for its cutting-edge proficiency in sensors. Our expertise is specifically applied in the domains of wearables, medical devices, and industrial and ...

based inRome, ITALY
MIR is a global medical device and software company founded in 1993, and today is present in more than 100 countries worldwide. For more than 25 years, we have been internationally recognized for our numerous innovations and advancements in three ...

based inSouth Hackensack, NEW YORK (USA)
Case Medical is a U.S.-based manufacturer specializing in medical instrument processing solutions aimed at improving healthcare quality, patient outcomes, and cost efficiency. They produce high-quality, cost-effective, and sustainable products used ...

based inParis, FRANCE
Qynapse, through QyScore, brings groundbreaking peace of mind to the global fight against CNS disease. Qyscore is a medical device software FDA-cleared – class II and CE-marked – class IIa (CE BSI). Qyscore is a medical device software FDA-cleared ...

based inTampa, FLORIDA (USA)
Qualityze is a cloud-based technology solution provider for Enterprise Quality Management System (QMS). We offer a comprehensive portfolio of high-quality, scalable, flexible, secure technology solutions for the critical quality process such as: ...

based inGivatayim, ISRAEL
Founded in 2005, Orcanos Software has built its reputation on delivering integrated solutions that ease the time-to-market product hurdles that are part of the Quality Journey. In fact, Orcanos is the only vendor that provides an integrated software ...
Our medical requirements management tool is perfect for manufacturers of medical devices and will provide you with a number of essential quality-related ...

based inBoston, MASSACHUSETTS (USA)
At PathAI, we’re dedicated to improving patient outcomes with reliable AI-Powered technology and meaningful collaboration with biopharma, laboratories, and clinicians — aiming to provide patients with access to accurate diagnoses and effective ...

based inChicago, ILLINOIS (USA)
Orthogonal is a product development and consulting firm that creates software for medical hardware such as smartphone apps talking to devices that operate directly on the human body to treat sickness and injury. Our solutions take on some of the ...
Human-centered Design and Development; Our focus on early and rapid user feedback leads to faster creation of medical device software products that engage ...

based inSan Jose, CALIFORNIA (USA)
Enhancing global compliance, creating a world where quality and compliance professionals, regulators, and government agencies come together to help the world comply with the intent and the spirit of laws, policies and mandates, ensuring continuous ...
This medical device software development, verification and validation training package of four courses covers wellIEC 62304 requirements and explains risk based approach for validation using ...