- Home
- Companies
- luxembourg
- in vitro diagnostic use
Refine by
In Vitro Diagnostic Use Suppliers Serving Luxembourg
41 companies found

based inPoway, CALIFORNIA (USA)
Diazyme uses its proprietary enzyme technologies to develop diagnostic reagents which can be used on most automated chemistry analyzers in user-friendly formats. Diazyme's products include test kits for diagnosis of cardiovascular disease, liver ...

based inQuilmes,, ARGENTINA
Diconex SA is a company dedicated to design, develop, produce and market clinical chemistry analyzers, used mainly by hospitals, clinics, veterinary clinics and laboratories, scientific research center, human laboratories, wine and food ...
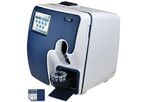
The Clinical Chemistry Autoanalyzer InCCA Counter 18sc is a measuring instrument and characterization of blood cells for in vitro diagnostic use, meassure 18 of the most important blood parameters. ...

based inCoralville, IOWA (USA)
A champion of life sciences research for more than 30 years, IDT develops and manufactures nucleic acid products that support the life sciences industry. Areas of focus include academic and commercial research, agriculture, medical diagnostics, ...
We have combined our proven oligo manufacturing expertise and ISO 13485 certified production processes to deliver GMP-manufactured MGB Eclipse Probes and companion primers. Together, these probe and primer pairs are ideal for genotyping qPCR assays. ...

based inHellerup, DENMARK
BioPorto offers high-quality monoclonal antibodies for pharmaceutical research and IVD testing in areas such as diabetes, obesity, and allergy. BioPorto is an in vitro diagnostics company focused on saving lives and improving the quality of life ...

based inVienna, AUSTRIA
ViennaLab Diagnostics specializes in easy-to-use in vitro diagnostic assays for the detection of genetic variants associated with genetic disorders, genetic predispositions, pharmacogenetics, cancer, and the human microbiome. Our focus is on product ...
A reliable tool to personalize therapy of lung cancer patients with acquired TKI-resistance mutation T790M in the EGFR ...

based inNY, NEW YORK (USA)
We are committed to developing new areas such as mitochondrial genome sequencing, gene mutation, comparative genomics and epigenetics, and provide highly customized services. Creative Biogene provides a comprehensive range of Separation & Extraction ...
This kit can label mitochondria in live cells with red fluorescence. The kit uses a proprietary dye that can accumulate in mitochondria based on the mitochondrial membrane potential gradient. The mitochondrial indicator is a hydrophobic compound ...

based inEpalinges, SWITZERLAND
At Abionic we are focused on delivering true patient-centric diagnosis solutions, which means a rapid, reliable test available at any time, from anywhere, whenever a patient needs it… A potential to save millions of lives is what drives us to work ...

based inNew York City, NEW YORK (USA)
Led by a team of experts in the fields of life sciences, oncology, pathology, technology, machine learning, and healthcare, Paige strives to transform cancer diagnostics. We make it possible not only to provide additional information from digital ...

based inWenzhou, CHINA
Global player specialized in manufacture and distributor of medical products, with a presence in 36 countries. A group with five (5) subsidiary factories and eight (8) joint venture manufacture base. A product scope more than 110 products, with a ...

based inBelgrade, SERBIA
Yunycom d.o.o. Belgrade was founded in 1990. as a private company and has been developing their business in the area of import, export and distribution of medical equipment, diagnostic materials and cosmetic products and services. Yunycom’s primary ...

based inPoway, CALIFORNIA (USA)
Located in San Diego County, California, an area well known for biotechnology and scientific discovery, CTK Biotech develops and manufactures innovative immunodiagnostic tools and point of care diagnostic test kits for the IVD community worldwide. ...
The RaFIA Immunofluorescence Analyzer is a fluorescence immunoassay analyzing instrument intended for use by healthcare professionals to aid in the diagnosis of conditions such as cardiovascular disease, pregnancy, infection, diabetes, renal injury ...

based inWaltham, MASSACHUSETTS (USA)
Nova Biomedical is a company dedicated to using advanced technology to develop better blood testing analyzers for medical use. These products are called in vitro diagnostic devices (IVD). We use some of the same advanced technology to provide the ...
Exceptional Simplicity in a New Generation Electrolyte Analyzer. Introducing a new generation electrolyte analyzer that combines the revolutionary microelectronics of the consumer world with new microsensor technology for a simpler, smaller, faster, ...

based inSan Diego, CA 92126 USA, CALIFORNIA (USA)
America Diagnostics, Inc. is a California company that develops and produces IVD devices and diagnostics raw materials. The quality of AmeriDx products is guaranteed by its quality management system. All the products are manufactured in a clean ...
This product is for in vitro diagnostic use, following guidance from the FDA for Emergency Use Authorizations of tests submitted for approval on March 16, 2020. This test has not ...

based inSydney, AUSTRALIA
SpeeDx specializes in molecular diagnostic solutions that go beyond simple detection to offer comprehensive information for improved patient management. SpeeDx is a dynamic, rapidly growing company with a strong portfolio of technology at the ...
Detect COVID-19 causative coronavirus, SARS-CoV-2; Multiplex RT-qPCR test with dual targets (RdRp/ORF1ab) and RNA-based internal control in a single-well. Scalable solution supporting surge ...

based inAmherst, NEW HAMPSHIRE (USA)
BUHLMANN Diagnostics Corp (BDC), Amherst NH is the North American affiliate of BÜHLMANN Laboratories AG, a leading worldwide operative manufacturer of ELISA kits, RIA kits, lateral flow assay kits, flow cytometry assay kits and turbidimetric assays ...

based inMinneapolis, MINNESOTA (USA)
Premier Biotech is a leading provider of rapid drug testing diagnostic devices backed by our CAP Forensic accredited laboratory located in Minneapolis, MN. Premier Biotech provides customized, drug screening services for our customers in criminal ...
The Premier Biotech COVID-19 IgG/IgM Rapid Test Cassette is a lateral flow immunochromatographic assay for the detection of SARS-CoV-2 antibodies in venous whole blood, serum or plasma. The COVID-19 IgG/IgM Rapid Test Cassette is intended for use ...

based inNewtown, AUSTRALIA
Genetic Signatures was founded in 2001 by the late Dr. Geoffrey Grigg. We are the developers of 3base technology which is the cornerstone of our EasyScreen Pathogen Detection Kits. Our proprietary technology provides hospital and pathology ...
The STI Genital Pathogen and Reproductive Health Reagents are rapid in vitro nucleic acid amplification assays for the qualitative detection of sexually transmitted pathogens as well as commensal and reproductive health-associated ...

based inThessaloniki, GREECE
Hellabio is a European Company established in 1986, located in Business Incubator Thermi, Thessaloniki, Greece. We are active researcher and manufacturer of innovative biological reagents for in vitro diagnostic use. We are committed to meeting and ...

based inSunnyvale, CALIFORNIA (USA)
JSR Life Sciences brings together the deep biochemical knowledge and understanding of human disease, expertise in drug discovery and development, and access to capital into a single, integrated framework that creates value for our customers. JSR ...
Magnosphere™ are magnetic microparticles which can be used for life science research and in vitro diagnostic use. Available are two different type of surface characteristics; ...

based inWhite City, OREGON (USA)
Biomed Diagnostics is the resulting collaboration of two physicians who found themselves vulnerable without the necessary equipment during a mercy mission in Central America. Without the necessary refrigeration, practical supplies and laboratory ...
Biomed Diagnostics' VTM-C19 Transit Tube is intended for on-site collection and transport to the testing laboratory of human clinical specimens containing SARS-CoV-2 — the virus that causes COVID-19 disease in humans and/or (USA only) ...