Show results for
Refine by
Nasal Swab Suppliers Serving Romania
38 companies found

based inXiamen, CHINA
About Biotime Established in April 2008, Xiamen Biotime is a hi-tech enterprise that specialized in R&D, production, and sales of POCT in vitro diagnostic devices and reagents. Biotime has successfully established the mature medical marketing ...
COVID-19 Ag & Flu A/B Combo Self Test is a colloidal gold immunochromatography intended for the in vitro rapid, simultaneous qualitative detection and differentiation of N protein from SARS-CoV-2, influenza A and influenza B directly from ...

based inSarasota, FLORIDA (USA)
Lumos Diagnostics specializes in rapid, cost-effective and complete point-of-care (POC) diagnostic test solutions to help healthcare professionals more accurately diagnose and manage medical conditions. Lumos offers customized assay development and ...
CoviDx™ SARS-CoV-2 Rapid Antigen Test is a lateral flow assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasal swabs and nasopharyngeal swabs ...

based inShenzhen, CHINA
LANDWIND MEDICAL is a health care group company headquartered in Shenzhen, China, established in 1994. Till now, we cater our global medical care to over 30,000 medical institutions in more than 130 countries. We integrate our dedicated and ...
Antigen detection can be used for early disease screening, the experiment is simple and rapid, suitable for large-scale detection, and can be used as a supplementary program for nucleic acid detection ...

based inVancouver, BRITISH COLUMBIA (CANADA)
Response Biomedical has a global distribution network and is focused on enabling our strong network of partners to be leaders in their markets by providing exceptional levels of service and support. Response Biomedical fosters a values-based ...
Response Biomedical provides a influenza test that aids in the rapid differential diagnosis of influenza viral infections in symptomatic patients. It identifies the presence of influenza A and influenza B nucleoprotein antigens in ...

based inGuangming District, CHINA
Located in Shenzhen, China, Dymind Biotechnology Co., Ltd. is a national-level high-tech enterprise specialized in the R&D, manufacture, sales and service of medical devices and reagents in the field of in-vitro diagnosis. Dymind is an enterprise ...

based inSeattle, WASHINGTON (USA)
InBios is a leading biotechnology company based in Seattle, Washington (USA). At InBios, we believe an accurate diagnosis is the first step to effective treatment. Specializing in the design, development and manufacture of immunodiagnostic devices ...
Smart DetectTM SARS-CoV-2 rRT-PCR Kit is a real-time RT-PCR test intended for the qualitative detection of nucleic acid from severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) in human nasopharyngeal swab, anterior ...

based inSomerset, NEW JERSEY (USA)
Even the most sophisticated, cutting-edge medical diagnostics aren’t of value unless we can get them to those who need them most. At Access Bio, we believe every life is precious, and every human on earth deserves the opportunity to live a healthy ...
The CareStart™ COVID-19 MDx RT-PCR is a real-time reverse transcription polymerase chain reaction (RT-PCR) test intended for the qualitative detection of nucleic acid from SARS-CoV-2 in respiratory specimens (such as nasopharyngeal, ...

based inTainan City, TAIWAN
Leadgene Biomedical Inc. is founded in 2013 and headquarters in Tainan, Taiwan. In the past years, we have become an all-around solution provider for proteomics study with more than thirty staff members from a three-scientists workshop, ...

based inWendelsheim, GERMANY
AESKU.GROUP, based in Wendelsheim, Germany, is a research-focused supplier of innovative and efficient products and services for the early detection, diagnosis and prognosis of autoimmune diseases. AESKU.DIAGNOSTICS is a leading manufacturer of ...
The AESKU.RAPID SARS-CoV-2 Rapid Test is based on immunochromatographic polymer technology combined with the sandwich principle for the qualitative detection of the nucleocapsid protein antigen in human nasal swab ...

based inSan Diego, CALIFORNIA (USA)
Raising the bar for over 80 years. QuidelOrtho is raising the bar in diagnostic care, from immunoassay and molecular testing to clinical chemistry and immunohematology, relentlessly pursuing a healthier future for all. Advancing diagnostics, ...

based inChengdu, CHINA
Maccura Biotechnology Co., Ltd. is a professional enterprise to research and manufacture clinical IVD products. Maccura was founded in 1994 and has been focusing on the research, manufacture, marketing, and services of IVD products. Maccura is a ...
Severe Acute Respiratory Syndrome Coronavirus 2(SARS-CoV-2) Antigen Assay Kit by Colloidal Gold ...

based inWhite City, OREGON (USA)
Biomed Diagnostics is the resulting collaboration of two physicians who found themselves vulnerable without the necessary equipment during a mercy mission in Central America. Without the necessary refrigeration, practical supplies and laboratory ...
Pediatric, Mid-Turbinate Flocked Nasal Swabs with Allium™ Flock Technology. Individually-sealed, sterile, flocked swabs with bear face shaped stopper and breakable shaft. For collecting SARS- ...

based inNanjing, CHINA
Founded in 2002, Jiji Biotech Co., Ltd.is a medical and health industry chain that integrates independent research and development, large-scale production and professional marketing. It has many subsidiaries and offices at home and abroad. the ...
Test Item: SARS-CoV-2 Antigen. Sample Type: Human nasal swab. Test Time: 15 min. Methodology: Immunofluorescence ...

based inSan Diego, CALIFORNIA (USA)
For over 23 years, ACON has led the way in making high quality diagnostic and medical devices more affordable to people all around the world. Headquartered in San Diego, California, the US office is the center of strategic management, ...
Critical Information When & Where You Need It; The Flowflex™ COVID-19 Antigen Home Test is all you need to determine your family’s Covid-19 status, whether symptoms are present or not. Can be used on children as young as 2 years old. ...

based inGuro-gu, SOUTH KOREA
NanoEntek possesses a key piece of Bio-MEMS technology that seamlessly integrates biotechnology with Micro-electro Mechanical Systems(MEMS), which is heralded as the most advanced technology in the 21st century. To realize this technology, ...
BUDDI™ is an image analyzer that evaluates the line signal intensities on the test ...

based inSuwon-si, SOUTH KOREA
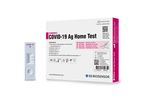
SD BIOSENSOR is a professional in-vitro diagnostics company with aims of contributing to higher life quality by diagnosing illnesses accurately and quickly. SD BIOSENSOR is researching and developing quick diagnostics, immunodiagnostics, molecular ...
STANDARD Q COVID-19 Ag Home Test is a rapid chromatographic immunoassay for the qualitative detection of SARS-CoV-2 nucleocapsid antigen present in human nasal ...

based inHercules, CALIFORNIA (USA)
Bio-Rad is a global leader in developing, manufacturing, and marketing a broad range of innovative products for the life science research and clinical diagnostic markets. With a focus on quality and customer service for over 65 years, our products ...

based inTrezzano Sul Naviglio (MI), ITALY
Richen Europe S.r.l. is a Pharmaceutical Company founded in 2014 as a subsidiary of the RICHEN MEDICAL SCIENCE GROUP and reference for the European market. We manufacture our own Medical Diagnostic and Medical Instruments focused on Gastroenterology ...
The rapid antigen test has become the most used tool – both publicly and privately – to quickly find out if you have come into contact with the Sars-CoV-2 virus, thus allowing better tracking of infections. ...

based inHangzhou, CHINA
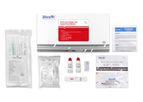
VivaChek Biotech (Hangzhou) Co.,Ltd was established in Hangzhou,China in August 2013.Since then, it becomes a reliable and rapidly growing manufacturer of POCT (point-of-caring testing) products and blood glucose monitoring systems with partners in ...

based inHauppauge, NEW YORK (USA)
Chembio is a leading diagnostics company focused on developing and commercializing point-of-care tests used to detect and diagnose infectious diseases, including sexually transmitted disease, insect vector and tropical disease, COVID-19 and other ...
Status Flu A & B is a CLIA Waived in vitro rapid qualitative test that detects Influenza Types A and B antigens directly from nasal or nasopharyngeal swab ...