- Home
- Companies
- usa south carolina
- delivery catheter
Show results for
Refine by
Delivery Catheter Suppliers Serving Usa South Carolina
29 companies found

based inIrvine, CALIFORNIA (USA)
JenaValve is a clinical-stage medical device company developing the first transcatheter heart valve technology that is uniquely designed for the minimally invasive treatment of both severe aortic regurgitation and stenosis. JenaValve is passionate ...
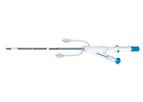
The JenaValve Transfemoral Delivery Catheter is designed to safely treat patients while delivering consistent, reproducible performance for physicians. The system features the ...

based inAlameda, CALIFORNIA (USA)
Penumbra, Inc., headquartered in Alameda, California, is a global healthcare company focused on innovative therapies. Penumbra designs, develops, manufactures and markets novel products and has a broad portfolio that addresses challenging medical ...
The Penumbra System MAX Reperfusion Catheters are intended for use in the revascularization of patients with acute ischemic stroke secondary to large vessel occlusion disease. The 3MAX and 4MAX Reperfusion Catheters feature advance tracking ...

based inSan Mateo, CALIFORNIA (USA)
Founded in 2015, Route 92 Medical is a privately held, commercial-stage medical technology company, based in San Mateo, CA. We are developing novel neuro-interventional technology with an application in stroke. Route 92 Medical’s mission is to ...
The Route 92 MedicalTenzing 7 Delivery Catheter is a single-lumen, variable stiffness catheter with a long, tapered tip delineated by two radiopaque markers. The Delivery ...

based inJiaxing City, CHINA
We, Best Hope Precision Device Co., Ltd. are engaged in design and manufacture precision injection molds, typically with rich experience for medical devices. A Leading Manufacturer of Molds and Injection Molded Parts for Medical Devices. In past ten ...
IV Infusion sets are used in devices that provide the proper volume of intravenous medicines, fluids etc. delivered at a certain flow rate. Basically, an IV Infusion set consists of long extruded tube, Connectors, Drip Chamber, Spike and Controller ...

based inTampa, FLORIDA (USA)
infuseon therapeutics, inc. was founded in 2012 by Cleveland Clinic for the purpose of developing its patented unique therapeutic delivery device, the Cleveland Multiport Catheter™. This multiport convection-enhanced delivery catheter was designed ...

based inSarstedt / Hannover, GERMANY
At MeKo, medical devices are manufactured to their perfection. Based on the designs of our customers the components are produced from tubing or sheet metals in compliance with the provided drawings and specifications. The manufacturing processes ...
We Laser Drill your Balloon: Drilled diameters down to 2 μm. Adjustable hole sizes in steps of 1 μm. Any desired hole distribution on the balloon. All kind of balloons: peripheral and coronary. No limitations in the balloon function: ...

based inOaks, PENNSYLVANIA (USA)
VascuTech Medical, LLC has evolved from a consulting firm serving major customers such as C.R. Bard, to a contract manufacturer of FDA and ISO 13485 registered medical device manufacturer serving OEMs and healthcare providers in interventional ...
Intravascular Drug Delivery Catheters are intended for the local delivery of various therapeutic and diagnostic agents into the coronary and peripheral vasculature. VascuTech Medical is a leading ...

based inFoxborough, MASSACHUSETTS (USA)
Viant is a global design and manufacturing services provider for the medical device industry, specializing in surgical technologies, orthopedics, cardiac & interventional, bioelectronics, drug delivery, and diagnostics & patient care. From design ...
Complex interventional devices. Steerable, balloon, and RF ablation catheters. Closure devices (femoral arterial and venous access). Introducers for transradial artery access. ...

based inHuntington, NEW YORK (USA)
Thoracent specializes in providing pulmonary and thoracic products to the U.S. medical market. The BONASTENT tracheobronchial stent is our flagship product. The BONASTENT stent features a nitinol woven design that is fully covered with an ultra-slim ...
The BONASTENT tracheobronchial stent incorporates the patented “hook & cross” nitinol stent design with a silicone cover purposely placed across the entire interior of the stent to prevent in-growth. The stent is delivered with an ...

based inIrvine, CALIFORNIA (USA)
Edwards Lifesciences is the global leader in the science of heart valves and hemodynamic monitoring. Driven by a passion to help patients, the company partners with clinicians to develop innovative technologies in the areas of structural heart ...
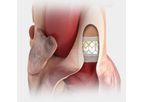
The first transcatheter heart valve approved for pre-stented transannular patches. Also approved for use in the pulmonic position in conduits and ...

based inSanta Clara, CALIFORNIA (USA)
EndoClot Plus, Inc. (EPI) is a privately held medical device company in Santa Clara, California. In collaboration with endoscopists and biomaterial scientists, EPI developed EndoClot Polysaccharide Hemostatic System (EndoClot PHS), a breakthrough ...
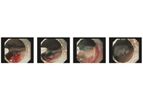
EndoClot PHS is a single use medical device consisting of Absorbable Modified Polymers (AMP) and a unique powder delivery system (applicator). It is a safe and effective method to control bleeding in both upper and lower GI tracts. It works ...

based inKennesaw, GEORGIA (US) (USA)
Our purpose is to develop simple, elegant solutions that address cardiac and vascular surgeons’ most difficult challenges in treating patients with diseases of the aorta and to deliver breakthrough technologies of unsurpassed quality that have ...
The E-tegra stent graft system enables the treatment of even difficult vascular anatomies and expands endovascular treatment options for infrarenal abdominal aortic ...

based inOakville, ONTARIO (CANADA)
CHS Ltd. (est. 1967) is a privately held medical device manufacturer and specialty distributor located in Oakville, Ontario. Customers served are in the acute hospital and non-acute healthcare space in Canada and Internationally. CHS ...

based inAliso Viejo, CALIFORNIA (USA)
In 1997 MicroVention was founded and incorporated in a small facility in Southern California. We are a worldwide community of over 2000 engineers, chemists, marketers, manufacturers, researchers, and innovators who share the same ethos - creating ...
Carotid Artery Stent Designed for Sustained Embolic ...

based inLake Forest, CALIFORNIA (USA)
NaviGate Cardiac Structures, Inc., (NCSI) is an early-stage company focused on developing transcatheter solutions for the treatment of atrioventricular valve regurgitation. The NaviGate valve-based technology was licensed from Cleveland Clinic and ...
The GATE™ System is intended to treat functional tricuspid regurgitation (FTR)/tricuspid insufficiency by replacing the function of a patient’s dysfunctional native tricuspid valve with a bio-prosthetic atrioventricular valved stent ...

based inOldsmar, FLORIDA (USA)
MicroLumen has been a leading manufacturer of high performance medical products since 1987. Our high performance medical tubing is used in a wide range of minimally invasive, critical OEM applications such as cardiovascular catheters, stent delivery ...

based inPrairie Village, KANSAS (USA)
At Artio, we are committed to developing and commercializing novel, best-in-class medical devices that have the potential to provide better patient outcomes. Delivering innovative medical devices with the potential to provide impactful solutions to ...
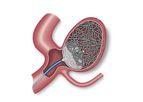
A combination of coils and an accessory balloon that has the potential to provide immediate cerebral aneurysm occlusion. Consists of an Endura Embolization Device, Endura Aneurysm Coils, and Endura Balloon ...

based inScottsdale, ARIZONA (USA)
Confluent Medical Technologies is dedicated to working collaboratively with our customers, taking their projects from rapid prototype into high volume production. Our unparalleled technical expertise, proven experience and partnership with our ...

based inBrea, CALIFORNIA (USA)
Established in 1997, Life Science Outsourcing, Inc. was founded to offer contract manufacturing services to emerging medical device companies. As an FDA-registered and ISO 13485-certified Contract Manufacturing Organization, we focus on assembly, ...
Life Science Outsourcing offers turnkey medical device manufacturing and assembly services that can be combined with our engineering and process development solutions to create a streamlined and comprehensive process. ...

based inWinsen, GERMANY
QualiMed was found in 1997 as an OEM manufacturer for implantable medical devices with a focus on the development and regulatory approval of coronary stents and their respective delivery devices. Later the business was expanded to peripheral ...
The NAVALIS-ppADVANCE Peripheral Vascular Self-Expanding Stent System combines highly durable nitinol stent with a flexible delivery system to provide proven clinical ...