- Home
- Companies
- usa wyoming
- delivery catheter
Show results for
Refine by
Delivery Catheter Suppliers Serving Usa Wyoming
24 companies found

based inOakville, ONTARIO (CANADA)
CHS Ltd. (est. 1967) is a privately held medical device manufacturer and specialty distributor located in Oakville, Ontario. Customers served are in the acute hospital and non-acute healthcare space in Canada and Internationally. CHS ...

based inIrvine, CALIFORNIA (USA)
Edwards Lifesciences is the global leader in the science of heart valves and hemodynamic monitoring. Driven by a passion to help patients, the company partners with clinicians to develop innovative technologies in the areas of structural heart ...
The first transcatheter heart valve approved for pre-stented transannular patches. Also approved for use in the pulmonic position in conduits and ...

based inCarlsbad, CALIFORNIA (USA)
PFM Medical, Inc. was established in 2001 as a subsidiary of pfm medical ag (Cologne, Germany), to serve as the headquarters for North America. PFM Medical, Inc. to not only meet, but exceed customer expectations and requirements; to design and ...
VETA Dialysis Catheters are side ported coiled silicone catheters with single and dual retention cuffs to provide tissue in-growth and anchor the catheter in place. With large spiral holes on the ...

based inBrea, CALIFORNIA (USA)
Established in 1997, Life Science Outsourcing, Inc. was founded to offer contract manufacturing services to emerging medical device companies. As an FDA-registered and ISO 13485-certified Contract Manufacturing Organization, we focus on assembly, ...
Life Science Outsourcing offers turnkey medical device manufacturing and assembly services that can be combined with our engineering and process development solutions to create a streamlined and comprehensive process. ...

based inOaks, PENNSYLVANIA (USA)
VascuTech Medical, LLC has evolved from a consulting firm serving major customers such as C.R. Bard, to a contract manufacturer of FDA and ISO 13485 registered medical device manufacturer serving OEMs and healthcare providers in interventional ...
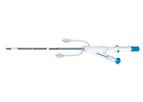
Intravascular Drug Delivery Catheters are intended for the local delivery of various therapeutic and diagnostic agents into the coronary and peripheral vasculature. VascuTech Medical is a leading ...

based inOldsmar, FLORIDA (USA)
MicroLumen has been a leading manufacturer of high performance medical products since 1987. Our high performance medical tubing is used in a wide range of minimally invasive, critical OEM applications such as cardiovascular catheters, stent delivery ...

based inWaltham, MASSACHUSETTS (USA)
Thermedical is developing a proprietary thermal ablation therapy that can treat significantly larger tissue volumes using a single device than conventional technology. Thermedical is a privately held medical device company based in Waltham, ...

based inAlameda, CALIFORNIA (USA)
Penumbra, Inc., headquartered in Alameda, California, is a global healthcare company focused on innovative therapies. Penumbra designs, develops, manufactures and markets novel products and has a broad portfolio that addresses challenging medical ...
The Penumbra System MAX Reperfusion Catheters are intended for use in the revascularization of patients with acute ischemic stroke secondary to large vessel occlusion disease. The 3MAX and 4MAX Reperfusion Catheters feature advance tracking ...

based inMontreal, QUEBEC (CANADA)
QXMedical was founded in 2007 in Montreal, Canada on the principle of providing uncompromised value and quality in underserved high-growth segments of the medical device market. Today, the company is known for its innovative solutions for complex, ...

based inReutlingen, GERMANY
It is probably a combination of many factors that has made us successful since 2004: the good collaboration of a medium-sized family company; the site in the heart of Germany’s medical technology region around the University of Tübingen; our ...
The semi-flexible hybrid cell Cobalt Chromium bare stent has been specifically designed for the effective treatment of Coarctation. Available in XL and ...

based inAliso Viejo, CALIFORNIA (USA)
In 1997 MicroVention was founded and incorporated in a small facility in Southern California. We are a worldwide community of over 2000 engineers, chemists, marketers, manufacturers, researchers, and innovators who share the same ethos - creating ...
Carotid Artery Stent Designed for Sustained Embolic ...

based inKennesaw, GEORGIA (US) (USA)
Our purpose is to develop simple, elegant solutions that address cardiac and vascular surgeons’ most difficult challenges in treating patients with diseases of the aorta and to deliver breakthrough technologies of unsurpassed quality that have ...
The E-tegra stent graft system enables the treatment of even difficult vascular anatomies and expands endovascular treatment options for infrarenal abdominal aortic ...

based inScottsdale, ARIZONA (USA)
Confluent Medical Technologies is dedicated to working collaboratively with our customers, taking their projects from rapid prototype into high volume production. Our unparalleled technical expertise, proven experience and partnership with our ...

based inRedwood City, CALIFORNIA (USA)
A global leader in interventional pulmonology, planning tools, and treatments for obstructive lung disease. Based in Redwood City, California, and Neuchâtel, Switzerland, Pulmonx is the maker of the Zephyr Endobronchial Valve. The Zephyr® Valve was ...
Sizing and Placement in One Step: Easy to use. Quick and accurate sizing of the length and width of the airways. Equivalent catheter sizes to valve sizes. Available in a “J” configuration to allow the bronchoscope access to more ...

based inCarlsbad, CALIFORNIA (USA)
CeloNova BioSciences, Inc. is developer and manufacturer of a portfolio of novel surfaced coated technologies, including the proprietary Polyzene-F nanocoating. This next-generation nanocoating is the result of years of rigorous scientific research ...

based inWinsen, GERMANY
amg International GmbH develops, manufactures and sells on a world-wide basis stents, stent implantation systems and balloon catheters as well as auxiliary products such as guide wires and inflation devices, which are used primarily in the field of ...
The ARTHOSPico is a multicellular Cobalt-Chromium stent that offers strength, stability, and flexibility for treating the most difficult lesions. The Co-Cr Stent is indicated for patients with coronary heart disease. It is inserted in coronary ...

based inIrvine, CALIFORNIA (USA)
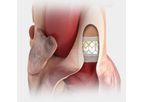
JenaValve is a clinical-stage medical device company developing the first transcatheter heart valve technology that is uniquely designed for the minimally invasive treatment of both severe aortic regurgitation and stenosis. JenaValve is passionate ...
The JenaValve Transfemoral Delivery Catheter is designed to safely treat patients while delivering consistent, reproducible performance for physicians. The system features the ...

based inWinsen, GERMANY
Stron Medical was launched in 2010 as a product line of QualiMed. With an impressive range of innovative products and a committed team of sales professionals, Stron Medical provides end-to-end solutions within Germany. QualiMed was found in 1997 as ...
The POLARIS-ppADVANCE Peripheral Vascular Self-Expanding Stent System combines highly durable nitinol stent with a flexible delivery system to provide proven clinical ...

based inWinsen, GERMANY
QualiMed was found in 1997 as an OEM manufacturer for implantable medical devices with a focus on the development and regulatory approval of coronary stents and their respective delivery devices. Later the business was expanded to peripheral ...
The NAVALISADVANCE Peripheral Vascular Self-Expanding Stent System combines highly durable nitinol stent with a flexible delivery system to provide proven clinical ...

based inLake Forest, CALIFORNIA (USA)
NaviGate Cardiac Structures, Inc., (NCSI) is an early-stage company focused on developing transcatheter solutions for the treatment of atrioventricular valve regurgitation. The NaviGate valve-based technology was licensed from Cleveland Clinic and ...
The GATE™ System is intended to treat functional tricuspid regurgitation (FTR)/tricuspid insufficiency by replacing the function of a patient’s dysfunctional native tricuspid valve with a bio-prosthetic atrioventricular valved stent ...