spinal-fusion-system Downloads
4 downloads found
Influx Sponge is a demineralized bone matrix (DBM) derived from 100% cancellous bone. A patented form provides a natural scaffold for cellular ingrowth and exposes bone-growth-inducing proteins to the healing environment. Influx Sponge is offered in strip, block, and disc forms. Due to Influx™ Sponge’s shape memory and malleable properties, each graft form has the ability to fill ...
The CervicalStim device is the only bone growth stimulation therapy approved by the FDA as a noninvasive, adjunctive treatment option for cervical fusion in patients at high-risk for non-fusion. The device uses a pulsed electromagnetic field (PEMF) to induce a low-level electrical field at the fusion site which stimulates bone healing at a molecular, cellular, and tissue level. With an overall ...
The SpinalStim device is FDA approved to be used after spinal fusion surgery or to be used to treat a failed fusion from a previous surgery. The devices stimulate the natural healing process of bone by sending low-level pulses of electromagnetic energy to the injury or fusion site. The device has an overall clinical success rate of 92% in treating spinal fusion surgery patients. In addition, the ...
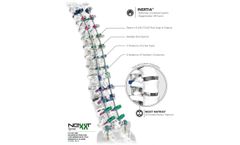
The Blade® Anterior Cervical Plate System is intended for anterior Screw fixation during the development of cervical spine fusion. The spinal fixation device includes Self-Drilling/Self-Tapping Screws, patented ‘Hurricane’ locking mechanism, and pre-contoured 1-5 level plates. Various instruments are also available as part of the Blade® Anterior Cervical Plate System for use ...