Refine by
Healing Process Articles & Analysis
52 news found
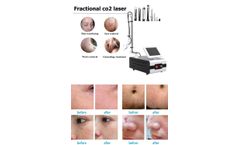
CO2 fractional laser equipment is a skin resurfacing treatment that uses targeted beams of light to vaporize the superficial layer of the skin. This process stimulates the growth of new collagen fibers, leading to firmer, smoother, and rejuvenated skin. It’s effective for addressing fine lines, wrinkles, acne scars, and areas of skin discoloration like sunspots and age spots. Bestview Laser ...
After the age of 30, elastin begins to disintegrate rapidly, and collagen is also rapidly lost. High-temperature HIFU treatment machine will damage the subcutaneous hyaluronic acid. Therefore, combined with treatment damage, the three core components will be disintegrated. If you do not take targeted nutrition, damage will occur. Cells are difficult to regenerate. We only have to do a good job of ...
The most commonly used fractional laser is CO2 fractional laser beauty machine. After more than ten years of clinical application, it can be said that CO2 fractional laser skin resurfacing treatment technology is relatively safe. As long as its therapeutic energy and action density are mastered, there are generally no obvious side effects of. Bvlaser is a professional CO2 fractional laser ...
BOC Sciences declares that some of its peptide products have been proven applicable for osteoporosis treatment, which is a significant contribution to the therapeutic areas. Bone tissue engineering and the research surrounding peptides have expanded significantly over the last few decades. Several peptides have been shown to support and stimulate the bone healing response and have been proposed ...
BRIM Biotechnology, Inc. (“BRIM,” TPEx 6885) is pleased to announce that it has received agreement from the US Food and Drug Administration (FDA) on the Phase 3 clinical trial design for its lead candidate for Dry Eye Disease (DED), BRM421, at the End of Phase 2 Meeting. BRIM will submit the Phase 3 study protocol to the FDA in Q4 2022. Dr. Haishan Jang, Chair and CEO ...
Roughly 8% of U.S. adults suffer from persistent or chronic back pain. That’s around 65 million people who are prevented from engaging in daily activities. Many are forced to miss days of work due to injury. With rising healthcare costs, many back pain sufferers think that they are left without a real solution, until they try epidural injections. An epidural injection is the infusion of ...
RevBio, Inc., announced that it has been awarded the opportunity to conduct an in vivo research experiment on the International Space Station U.S. National Laboratory (ISS National Lab). This experiment will examine the biomaterial's osteoconductivity when used in a microgravity environment where bone density and the ability to regenerate new bone tissue is significantly compromised. "Given the ...
Fractional laser is a commonly used instrument in clinical beauty medicine. In addition to treating acne scars, burn scars, and trauma scars, it also has a good effect on facial rejuvenation, freckle removal, skin shrinking, and stretch marks. Bvlaser is a CO2 fractional laser machine factory supplier, we have best fractional laser machine for sale. What is fractional laser Fractional laser ...
Milan, Italy (June 1st, 2022) – Today, KLISBio, a MedTech company challenging the status quo in regenerative medicine by leveraging a silk-based multidisciplinary technology platform, announced its attendance to 2022 IFSSH, IFSHT & FESSH Combined Congress organized by the International Federation of Societies for Surgery of the Hand and International Federation of Societies for Hand ...
ByKLISBio
SANUWAVE Health, Inc. (OTCQB:SNWV), a leading provider of next-generation wound care products and its JV partner Diversa S.A. have announced a new distribution agreement with Grupo Suprimed to market and sell the dermaPACE® System in the private healthcare market in Brazil. The dermaPACE® System is currently licensed or approved for advanced wound care indications in Brazil, Mexico and ...
SANUWAVE Health, Inc. (OTCQB:SNWV), a leading provider of next-generation advanced wound care medical systems for the repair and regeneration of skin and vascular structures and the research for new applications for shockwave systems in the non-medical field, announced today the patent activity for the full year 2020 and the first quarter of ...
REVA Medical, LLC. (“REVA” or the “Company”), a leader in bioresorbable polymer technologies for vascular applications is pleased to announce exceptional 5 Year clinical results from its FANTOM II Study. The trial evaluated the safety and performance of the Company’s Fantom sirolimus-eluting bioresorbable coronary scaffold in over 240 patients outside the United ...
BRIM Biotechnology, Inc. (“BRIM”) a clinical-stage company developing novel regenerative therapies in ophthalmology, today announces it has entered into a new strategic partnership with Ora®, Inc. (“ORA”) the world’s leading, ophthalmic research organization, for the late-stage clinical development of lead drug candidate, BRM421, for dry eye syndrome (DES). BRIM ...
Cerapedics Inc., an ortho-biologics company dedicated to enhancing the science of bone repair, today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device designation for its investigational P-15L Bone Graft for the treatment of degenerative disc disease (DDD). The FDA’s Breakthrough Device designation is designed to expedite the development and review ...
Cerapedics, a private ortho-biologics company, today announced the U.S. Food and Drug Administration (FDA) has approved the company’s Investigational Device Exemption (IDE) supplement, reducing the enrollment requirement for the on-going clinical trial, ASPIRE, for P-15L Bone Graft in a single level transforaminal lumbar interbody fusion (TLIF) surgery. “We are pleased to announce ...
Cerapedics, a private ortho-biologics company, today announced that results from a clinical trial evaluating i-FACTOR Peptide Enhanced Bone Graft in non-instrumented lumbar fusion surgery has been published in the May 2020, Volume 20, Issue 5 print of The Spine Journal as the lead article. The data demonstrate that elderly patients in Denmark treated with i-FACTOR bone graft plus local bone had a ...
SANUWAVE Health, Inc. (SNWV) focuses on the development and commercialization of innovative advanced wound care medical systems for the repair and regeneration of skin and vascular structures and the research for new applications of shockwave and ultrasound systems in the non-medical field, announced today the patent activity for the past year. For the shockwave technology, the following new ...
SANUWAVE Health, Inc. (SNWV), a leading provider of next-generation wound care products, today announced three new board members will join as one departs. “We are excited to expand our board with three new members who will help us reach new heights by bringing diverse expertise and insight to our work,” said SANUWAVE CEO & Chairman of the Board Kevin A. Richardson, II. “We ...
Biostage, Inc. (OTCQB: BSTG) ("Biostage" or the "Company"), a cell-therapy biotechnology company with successful first-in-human experience in treating esophageal cancer (conducted at the Mayo Clinic and published last August) and FDA approval to commence a clinical trial of the Biostage Esophageal Implant for severe esophageal disease including cancer, today announced that the U.S. Patent and ...
SANUWAVE Health, Inc. (SNWV), a leading provider of next-generation wound care products, has announced they will be updating shareholders on a monthly basis during 2022 with regards to their Sales Funnel and System Trials. This news comes simultaneously with the company’s announcement for growth through a medical non-wound care vertical market expansion. Both the Sales Funnel, defined as ...