Refine by
Drug Hd Safety Articles & Analysis
40 news found
Obtaining the Marketing Authorisation Application (MAA) is a key step in the commercialization of drugs in the EU, and also an important help to promote the global market expansion of pharmaceutical companies. The MAA is complicated by the need to consider both European economic integration and the independence of individual member states. As a specialist in regulatory matters, Proregulations has ...
Ace Therapeutics, a preclinical contract research provider dedicated to offering comprehensive one-stop services, released the expansion of its research capabilities in the area of depression through the introduction of comprehensive depression-related behavior tests in its preclinical investigation processes. This integral development aims to enhance the understanding of depressive disorders and ...
CD Formulation, an expert in the pharmaceutical research and development field, has announced the expansion of its comprehensive pharmacological services to support the burgeoning sector of veterinary drugs. The move is poised to elevate the standard and efficacy of veterinary pharmaceuticals, reinforcing CD Formulation’s dedication to the well-being of animals globally. “Our ...
CD Bioparticles, a leading manufacturer and supplier of numerous drug delivery products and services, has recently expanded its low PDI polymer portfolio and announces its new offering of Polyolefin Family polymers with a wide range of properties that make them well-suited for a variety of drug delivery applications. Low dispersion index polymers are polymeric compounds with low molecular weight ...
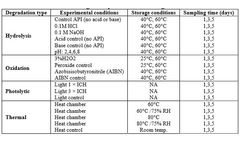
STEMart, a US-based provider of comprehensive services for all phases of medical device development, announced the launch of its new Force Degradation Services to help pharmaceutical and medical device companies evaluate the stability of their drug candidates and finished products under a variety of stress conditions, ensuring their safety and efficacy throughout their shelf life. Forced ...
BySTEMart
Alfa Chemistry serves as an emerging pharmaceutical research organization with a mission to revolutionize the field of preclinical drug research with its wide range of comprehensive services. With a commitment to quality and excellence, Alfa Chemistry offers pharmacological analysis, drug safety evaluation, pharmacokinetic analysis, and bioanalytical services to streamline the drug development ...
CD ComputaBio, a reliable computational biology service provider in New York, is committed to assisting research and trials, as well as providing access to the latest software, technologies, and expertise at competitive prices and with fast turnarounds for researchers. The company is pleased to announce the launch of the Protein-Small Molecule Docking service, designed to help customers quickly ...
The Microplate Reader Company – and InvivoSciences, Inc. – a leader in stem cell tissue engineering providing a novel solution in first-in-class drug discovery – announced today a strategic collaboration to market the time-dependent fluorescent assessment of engineered 3D heart tissues using advanced microplate reader technologies. Employing an automated system, 3D heart ...
Ezisurg Medical’s staplers have been approved by the Saudi Food and Drug Authority recently. This is the latest achievement made in Ezisurg Medical’s international sector, soon after finishing CE and FDA certification or ...
Destiny Pharma plc (AIM: DEST), a clinical stage innovative biotechnology company focused on the development of novel medicines that can prevent life-threatening infections, is pleased to announce the commencement of an Investigational New Drug (IND) enabling safety study with its novel XF-73 Dermal formulation. This study is the second of two planned preclinical safety studies of the XF-73 ...
XPOVIO® is the first and only exportin 1 (XPO1) inhibitor approved in Taiwan Antengene plans to submit for national health insurance reimbursement in Taiwan for XPOVIO® in Q4 2022 Shanghai and Hong Kong, PRC, October 21, 2022 — Antengene Corporation Limited (“Antengene” SEHK: 6996.HK), a leading innovative, commercial-stage global biopharmaceutical company ...
Gesynta Pharma AB today announces the decision to advance the development of its clinical-stage drug candidate GS-248 in endometriosis - a chronic inflammatory condition affecting approximately 10 percent of all women of reproductive age. This strategically important decision follows a recent preclinical proof-of-concept study with GS-248 in an advanced model of endometriosis, where ...
XPhyto Therapeutics Corp. (CSE:XPHY / OTC:XPHYF / FSE:4XT) (“XPhyto” or the “Company”) is pleased to report a significant potential market opportunity for its oral dissolvable (“ODF”) biosensor screening tests for oral inflammation. Certain buprenorphine medicines prescribed to treat opioid use disorder (“OUD”) and pain have been recently associated ...
Gesynta Pharma AB today announces results from an exploratory Phase II study of the candidate drug GS-248 in systemic sclerosis patients. GS-248 was well tolerated, exhibited a favorable safety profile, and elicited a potent systemic inhibition of the target enzyme mPGES-1. However, no significant effects on the patients' symptoms could be demonstrated. For this reason, the company intends to ...
Earlier this month, CD Formulation announces that it is now capable of providing nitrosamine impurities analysis service for customers who are involved with drug formulation projects and need help from CRO companies like CD Formulation. The analysis of nitrosamines can be challenging. These ultra-low levels of impurities must be quantified in a variety of complex matrices. Equipped with all the ...
“We have established an approach that transforms real-world, in-human treatment outcomes and safety data into extensive datasets to better characterize desirable and undesirable interactions between molecular pathways and drugs,” explains Dr. David Jackson, Chief Innovation Officer at Molecular Health and co-author on all three articles. “The studies demonstrate a standardized ...
Having a leading expert team specialized in pharmaceutical formulation development, CD Formulation recently announces its ability to achieve solubility improvement and bioavailability enhancement based on active pharmaceutical ingredient (API) characteristics and customer development goals. For researchers who are troubled by insoluble or poorly soluble drugs, CD Formulation is now prepared to ...
EG-Q won the '2022 Korea Consumer Preference No. 1' medical device (liquid band) category. '2022 Korea's No. 1 Consumer Preference' was established to provide consumers with an opportunity to receive verified services and the right selection criteria by discovering and awarding companies, products, and brands that have been verified through fair research. EG-Q is a brand specializing in ...
Theramx announced on the 14th that it has signed an annual export contract with the Madrid Regional Health Agency, Spain, and has completed the delivery of the product. The product exported to Spain is EG-Q Bond, a bioadhesive for epidermal bonding that joins the affected area without using a separate suture for 20,000 endoscopic and laparoscopic surgeries. A Theramx official said, ...
-SITC 2021, re-hosted face to face meeting for the first time in 2 years due to COVID-19…topic for overcoming tolerance of Immuno-oncology -International researchers pay attention to immuno-oncology GI-101’s material excellence that had the mechanism of overcoming tolerance GI Innovation revealed KEYNOTE-B59’s clinical research process of GI-101(CD80/IL-2 variant bispecific ...