Refine by
Medical Announces Product Testing Articles & Analysis
45 news found
STEMart, a US-based provider of comprehensive services for all phases of medical device development, announced the expansion of its service offerings to include the Bacterial Reverse Mutation Test (Ames) service. ...
BySTEMart
STEMart, a US-based provider of comprehensive services for all phases of medical device development, announces the expansion of its medical device testing capabilities and introduces Balloon Catheter Testing Services for the development of safe and effective medical devices. ...
BySTEMart
Bedfont® Scientific Ltd., world leaders in breath analysis with over 47 years of knowledge in designing and manufacturing medical breath analysis devices, welcomes the recent update to the National Institute for Health and Care Excellence (NICE) guidelines on asthma management. Bedfont® manufactures the NObreath® Fractional exhaled Nitric Oxide (FeNO) device, which aids in diagnosing ...
Planning brain surgery is a delicate process that requires precision to avoid damaging surrounding brain tissue and to prevent post-surgery complications. Synaptive Medical, a leader in medical technology, is set to enhance this process by evaluating its new software called Modus Plan, at Unity Health Toronto’s St. Michael’s Hospital, with support from OBIO’s Life Sciences ...
Protheragen-ING Lab, a well-known and reputable Good Laboratory Practices (GLP) service provider, has recently unveiled its cutting-edge medical device services. With a track record of excellence and reliability in providing high-quality services to the pharmaceutical and biotechnology industries, Protheragen-ING Lab is now expanding its offerings to include comprehensive solutions for medical ...
A breakthrough in CD formulation has enabled researchers to evaluate the efficacy of oral thin films, ensuring both therapeutic efficacy and safety. This new development has the potential to revolutionize the way oral medications are tested and administered, leading to more precise and effective treatments for a wide range of medical conditions. The development of oral thin films has gained ...
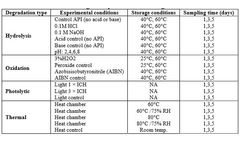
STEMart, a US-based provider of comprehensive services for all phases of medical device development, announced the launch of its new Force Degradation Services to help pharmaceutical and medical device companies evaluate the stability of their drug candidates and finished products under a variety of stress conditions, ensuring ...
BySTEMart
ViGeneron GmbH, a gene therapy company, announced the closing of its series A financing round led by WuXi AppTec and Sequoia Capital China. ...
Modular Medical, Inc. (the "Company" or "Modular Medical") (NASDAQ:MODD), a development stage, insulin delivery technology company seeking to launch the next generation of easy to use and affordable insulin pump technology, today announced an update on the development of its initial insulin pump product. The Company has ...
The 101 Corridor is leading the way in new diagnostics that will bring information to you and your doctor within minutes or hours. You will no longer have to wait for days for a central diagnostic laboratory to provide critical medical test data regarding your health. This program showcases a range of young diagnostic companies that are poised to launch exciting products and services that will ...
(NASDAQ: CING), a biopharmaceutical company utilizing its proprietary Precision Timed Release™ (PTR™) drug delivery platform technology to build and advance a pipeline of next-generation pharmaceutical products, today announced it has executed a Master Services Agreement (MSA) with Societal CDMO, Inc. ...
XPhyto Therapeutics Corp. (CSE:XPHY / OTC:XPHYF / FSE:4XT) (“XPhyto” or the “Company”) is pleased to report that it has signed a non-binding Letter of Intent (“LOI”) to identify and assess potential business synergies for manufacturing, import/export, distribution and product development with a US-based thin film manufacturing firm (the “Firm”). ...
XPhyto Therapeutics Corp. (CSE:XPHY / OTC:XPHYF / FSE:4XT) (“XPhyto” or the “Company”) is pleased to report a significant potential market opportunity for its oral dissolvable (“ODF”) biosensor screening tests for oral inflammation. Certain buprenorphine medicines prescribed to treat opioid use disorder (“OUD”) and pain have been recently associated ...
Telehealth use skyrocketed in the early months of the Covid-19 pandemic when many health providers were forced to deliver services remotely. Although telehealth visits have dropped from their peak in 2020, they remain elevated even as many patients return to in-person care, according to the results of an analysis by Epic Research and the Kaiser Family Foundation. Survey results show that ...
Why should payers care about clinical laboratory testing? Clinical laboratory testing plays an essential role in the delivery of health care. From early detection and diagnosis of disease to individualized treatment plans based on a person’s unique biology, medical care depends on accurate and timely health data. Seventy percent of medical decisions depend on lab test results, so making ...
Pulmonx Corporation (Nasdaq: LUNG) (“Pulmonx”), a global leader in minimally invasive treatments for severe lung disease, announces the presentation of interim results from the CONVERT Study at the 2022 European Respiratory Society (ERS) International Conference. Data on the first 40 patients in the study demonstrated that treatment with the AeriSeal System successfully converted the ...
“Babson is working with pharmacies to offer medically accurate blood testing that is easier, more convenient and less invasive than traditional ...
STEMart, a provider of CRO services dedicated to integrated medical device and diagnostic clinical development, introduced new Antibiotic Potency Tests to assess the bioactivity or potency of various antibiotics for medical devices. The development of these new tests strictly follows the Antibiotics-Microbial Assays in USP General Chapter 81 to ensure the highest efficacy. Microbial potency ...
BySTEMart
Liaison Memed Bv is an immune system based protein signature test for distinguishing between bacterial and viral infections The test was developed following the licensing agreement signed with Memed as announced in September 2020 The new test is available on the Liaison Xl platforms in countries that accept CE mark and U.S. ...
ByMeMed
The Body Agency Collective, Visby Medical, Inc. and CCHCI observe National STD Awareness Month with women’s health initiative on US-Mexico border. CCHCI among first healthcare providers in the nation to adopt new handheld PCR technology to detect gonorrhea, chlamydia and trichomoniasis in a single visit. Chiricahua Community Health Centers, Inc. (CCHCI) today announced a collaboration ...