Refine by
Safety Allroundline With Safety Device Articles & Analysis
73 news found
STEMart, a US-based provider of comprehensive services for all phases of medical device development, announced the expansion of its service offerings to include the Bacterial Reverse Mutation Test (Ames) service. This service provides manufacturers and researchers a rapid, cost-effective, and robust method for assessing the mutagenic potential of new chemicals and leachable substances from ...
BySTEMart
Introduction The pharmaceutical industry demands absolute precision, reliability, and safety throughout every stage of production. Whether manufacturing life-saving medicines or performing sensitive research, facilities must maintain strict control over environmental conditions. One often overlooked but essential component is gas analysis. Gases such as oxygen, nitrogen, and carbon dioxide play ...
STEMart, a US-based provider of comprehensive services for all phases of medical device development, announces the expansion of its medical device testing capabilities and introduces Balloon Catheter Testing Services for the development of safe and effective medical devices. These reliable testing solutions will meet the evolving needs of the medical device industry and help manufacturers ...
BySTEMart
A Centers for Disease Control and Prevention (CDC) report published on October 3, 2024, linked Burkholderia multivorans infections to ice machines in multiple healthcare facilities in the western United States. This post summarizes key findings, implications, and recommended practices for healthcare providers to ensure patient ...
Huateng Pharma, a leading supplier of PEG derivatives and trusted CDMO partner, is thrilled to announce its participation in CPhI Worldwide 2024, scheduled for October 14-16 in Milan, Italy. Attendees are encouraged to visit booth #3F81, where the Huateng team will present its innovative capabilities in GMP-grade PEG derivative production, Contract Development and Manufacturing Organization ...
Protheragen-ING Lab, a well-known and reputable Good Laboratory Practices (GLP) service provider, has recently unveiled its cutting-edge medical device services. With a track record of excellence and reliability in providing high-quality services to the pharmaceutical and biotechnology industries, Protheragen-ING Lab is now expanding its offerings to include comprehensive solutions for medical ...
Boston: “According to the latest BCC Research study, the demand for EMI/RFI: Materials and Technologies is estimated to increase from $7.4 billion in 2023 to reach $9.2 billion by 2028, at a compound annual growth rate (CAGR) of 4.6% from 2023 through 2028.” This comprehensive report delves into the dynamic landscape of the EMI/RFI materials and technologies market, meticulously ...
Alfa Chemistry serves as an emerging pharmaceutical research organization with a mission to revolutionize the field of preclinical drug research with its wide range of comprehensive services. With a commitment to quality and excellence, Alfa Chemistry offers pharmacological analysis, drug safety evaluation, pharmacokinetic analysis, and bioanalytical services to streamline the drug development ...
TOMI Environmental Solutions, Inc.® ("TOMI") (NASDAQ: TOMZ), a global company specializing in disinfection and decontamination solutions, today announced that the Company has entered into a contract with Vizient, Inc. increasing TO Mi's presence in the U.S. healthcare system. Vizient is the largest group purchasing organization (GPO) in the healthcare industry supplying around $100 billion in ...
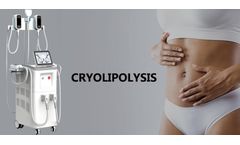
Cryolipolysis is a medical aesthetic technique that uses freezing to break down excess fat in the body to achieve a slimming effect. This technique has a great popularity and many people feel confident and beautiful because of the success of this technique to lose weight. As we all know, the medical aesthetic industry has always been a hot industry and cryolipolysis beauty machine as a technique ...
Castle Biosciences, Inc. (Nasdaq: CSTL), a company improving health through innovative tests that guide patient care, today announced that the Accreditation Committee of the College of American Pathologists (CAP) has accredited its clinical laboratory facility in Pittsburgh. This achievement follows a recent on-site inspection as part of the CAP’s Laboratory Accreditation Program. ...
Thaw More Plasma Faster with ZipThaw® Traditionally, plasma thawing is done in hot water baths which are slow, messy, and outdated. With their slow thawing to imprecise temperatures, time taken for warm up, and extensive maintenance and clean up needs, water baths make it impossible to maintain high plasma throughput levels. Now there’s an easier way to thaw more plasma, faster. ...
Recommendation is based on the Phase III study FIREFLEYE and data from the follow-up study FIREFLEYE NEXT Retinopathy of prematurity (ROP) can lead to severe visual impairment and blindness Bayer will apply for a patent term extension for the patent covering aflibercept, the active ingredient in Eylea™, of six months once the European Commission adopts a decision for a label extension ...
ByBayer AG
Inari Medical, Inc. (NASDAQ: NARI) (“Inari”) a medical device company focused on developing products to treat and transform the lives of patients suffering from venous and other diseases, announced three new data sets will be presented during Late-Breaking Clinical Trial sessions at the 2022 TCT (Transcatheter Cardiovascular Therapeutics) and the 2022 VEINS (Venous Endovascular ...
Molecular Health, an international biotech IT company based in Heidelberg, Germany, has received European Union certification for its MH Guide clinical decision support software (SaaS) under the new In Vitro Diagnostics Regulation (EU) 2017/746 (IVDR). The MH Guide software, which is used in molecular pathology laboratories, is the first of its kind in Europe to receive this certification. The ...
ATLANTA, Georgia – The early success of Linear Health Sciences in developing its Orchid™ Safety Release Valve to prevent unwanted dislodgement of IV catheters was highlighted in a panel discussion at the recent annual conference of the Southeastern Medical Device Association (SEMDA). Dan Clark, co-founder and Chief Marketing Officer of Linear Health Sciences, represented the company ...
OKLAHOMA CITY, Oklahoma – Linear Health Sciences, developer of the Orchid™ Safety Release Valve (SRV), announced that it has received a substantial grant to work with the Global Center for Medical Innovation. GCMI is a comprehensive medical-device innovation center that guides the development and commercialization of new medical devices. Linear Health Sciences is the first company to ...
OKLAHOMA CITY – Linear Health Sciences today announced that the United States Patent and Trademark Office has issued the company’s first patent related to its flagship product, the Orchid Safety Release Valve™. US Patent No. 9,861,805 B2 covers wide-ranging claims behind the needless connector for use in medical access devices such as IV catheters. “The patent issuance ...
Portsmouth, N.H. – IV dislodgement is a near-daily problem for clinicians and their patients, according to a recent focus group of vascular access experts. The clinicians also reported there is a need for better technology to mitigate the potentially serious consequences of frequent IV dislodgements. “This research discussion addressed a frustrating problem that those of us in ...
Reflow Medical, Inc., a California-based medical device company, has completed enrollment in the DEEPER LIMUS clinical trial (NCT04162418) to evaluate the Temporary Spur Stent System, a patented device designed to treat long, diffuse and severely calcified infrapopliteal disease. The system allows for uniform expansion of the stent to maximize lumen diameter and may reduce acute vessel recoil ...