Refine by
Therapy Unit Articles & Analysis: Older
175 news found
CD Formulation, a reputable contract research organization (CRO) based in New York, has become a trusted partner for many pharmaceutical companies around the world seeking innovative drug formulation methods and solutions. Recently, CD Formulation further strengthened its role as a drug delivery expert by demonstrating its expertise in the development of cutting-edge CAR-T and CAR-NK cells for ...
Healiva®, a patient-centric company delivering life-enhancing precision medicine for patients with chronic and acute wounds, announced today the acquisition of two innovative cell therapy assets from Smith+Nephew (LSE:SN, NYSE:SNN), the global medical technology business. The acquisition enables Healiva® to establish one of the world’s broadest portfolios of affordable, ...
Shanghai and Hong Kong, PRC, December 23, 2022 — Antengene Corporation Limited (“Antengene” SEHK: 6996.HK), a leading innovative, commercial-stage global biopharmaceutical company dedicated to discovering, developing and commercializing first-in-class and/or best-in-class therapeutics in hematology and oncology, today announced that it has submitted New Drug Applications ...
Congratulations to the ONWARD team for the incredible progress they are making in the field of neuroengineering! In recent days, numerous media have reported the news of a great advance for science, and especially for all those people with spinal cord injury and their medical and caregiver teams. The ONWARD team has developed an epidural electrical stimulation system using electrodes placed ...
Castle Biosciences, Inc. (Nasdaq: CSTL), a company improving health through innovative tests that guide patient care, today announced that the Accreditation Committee of the College of American Pathologists (CAP) has accredited its clinical laboratory facility in Pittsburgh. This achievement follows a recent on-site inspection as part of the CAP’s Laboratory Accreditation Program. ...
An objective response rate (ORR) of 52.4% was observed in relapsed or metastatic cervical cancer in Phase I/II TORCH-2 Study of ATG-008 (onatasertib) in combination with toripalimab, regardless of PD-L1 status Study builds on promising results of Phase II TORCH monotherapy study in HBV+ patients with unresectable hepatocellular carcinoma (HCC) which demonstrated an ORR of 16.7% in the 45 mg ...
BOSTON & LJUBLJANA, Slovenia--(BUSINESS WIRE)--Neutron Therapeutics, a targeted radiation therapy company developing a comprehensive solution for Boron Neutron Capture Therapy (BNCT), and Cosylab, the world's leading provider of control systems for the planet's most complex machines, today announced that they have reached their second joint milestone in the clinical commissioning of Neutron ...
Virica Biotech Inc. (“Virica”), a leading developer of solutions for scaling of viral medicines, will be featured in a live webinar to be held on Monday, November 14, 2022, at 2:30pm EST. This webinar will focus on process intensification in the manufacturing of adeno associated virus (AAV) vectors. AAV vectors are critical to gene therapy development. These vectors are the delivery ...
XPOVIO® is the world’s first oral selective inhibitor of the nuclear export protein XPO1, with regulatory approvals in 13 countries and regions including the United States, Israel, the United Kingdom, the European Union, Canada, Norway, Iceland, Lichtenstein, South Korea, Mainland China, Taiwan,China, Singapore and Australia. ...
Today, the Baker-Polito Administration announced that more than $1.5 million has been awarded to four projects that seek to increase testing capacity and provide solutions for coronavirus testing. The funding is through the Accelerating Coronavirus Testing Solutions (A.C.T.S.) Program, designed and administered by the Massachusetts Life Sciences Center, which supports projects focused on two core ...
Myrtelle Inc., (Myrtelle), a clinical stage gene therapy company focused on developing transformative treatments for neurodegenerative diseases, and Forge Biologics, a gene therapy-focused contract development and manufacturing organization, today announced a manufacturing partnership that will advance Myrtelle’s novel gene therapy for monogenic hearing loss, Myr-201, into clinical trials ...
Anuncia Medical, Inc., announced that the U.S. Food and Drug Administration (FDA) cleared the ReFlow® System Mini for the treatment of patients with hydrocephalus and other cerebrospinal fluid (CSF) disorders that require shunting. Anuncia Medical announced FDA clearance of ReFlow® System Mini for the treatment of cerebrospinal fluid (CSF) disorders. The FDA 510(k) clearance allows ...
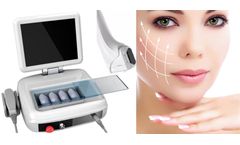
HIFU is the abbreviation of high intensity focused ultrasound. It uses focused ultrasound energy to accurately inject the dermis, subcutaneous tissue, and SMAS (facial superficial aponeurotic system) fascia layer of the skin to increase the temperature of the target site and initiate collagen regeneration and firming. There are two keys to HIFU skin tightening machine treatment: depth and ...
ABK Biomedical, Inc., an innovative medical device company dedicated to the research, development, and commercialization of advanced imageable embolic therapies, announces FDA 510(k) clearance of Easi-Vue embolic microspheres for the treatment of patients suffering from arteriovenous malformations and hypervascular tumors. Arteriovenous malformations (AVMs) occur when capillaries, veins, and ...
The clinical trials conducted by La Rioja’s healthcare service demonstrates the effectiveness of the only therapeutic massage robotic device that uses pressurized air to treat back pain. ...
Aatru Medical, LLC ("Aatru") today announced U.S. Food and Drug Administration (FDA) 510(k) Class II clearance of the NPSIMS™ Negative Pressure Surgical Incision Management System. The NPSIMS utilizes an innovative mode-of-action that eliminates the expensive electromechanical pump, battery, and electronics found in most other negative pressure wound therapy (NPWT) systems being deployed ...
” UK 1st National Health Service (NHS) EndoBarrier Service for Uncontrolled Diabesity In the first NHS EndoBarrier service in the U.K., EndoBarrier was provided to patients with uncontrolled diabesity despite all available standard therapies and monitored in the ABCD Worldwide Registry. Data presented at ADA was for 4-year outcomes for all 62 treated patients, of these 44 ...
Santa Clara, Calif.– July 12, 2022 – Ancora Heart, Inc., a company developing a novel device-based therapy to address heart failure, today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation to the AccuCinch® Ventricular Restoration System. Currently being evaluated in the CORCINCH-HF pivotal clinical trial, the AccuCinch System ...
“It’s an exciting time for gamma-delta T cell therapies as big pharma enters the field,” said Dr. LaMontagne. “My career has always been directed toward advancing novel disruptive therapies. ...
In an exciting report by ZDF, it is shown how CUREO can be used as a therapy method for Long Covid patients. In addition to acutely affected covid patients, many people struggle with the long-term consequences of the disease. These can be treated efficiently with CUREO. In addition to acutely affected Covid patients, many people also have to struggle with the long-term consequences of the virus. ...