Toxicity Testing Articles & Analysis
17 news found
Molecular Devices, LLC., a leading provider of high-performance life science solutions, today announced the acquisition of Cellesce Ltd (“Cellesce”) which specializes in contract development and manufacturing of large scale patient-derived organoids (PDOs) for diverse applications, including drug screening. Drug efficacy and toxicity testing often ...
Its PROTAC in vivo evaluation services encompass general studies of toxicology and in vivo pharmacokinetics, tests of allergy and in vitro hemolysis, as well as immunotoxicology analysis. ...
Human induced pluripotent stem cell-derived beta-cells for drug and toxicity ...
In a letter to the Company dated May 13, 2022, the FDA stated that they were upholding their decision that the device is not substantially equivalent for extended use based on their analysis of the methodology used for exhaustive extraction testing. The FDA also stated that the Company may submit a new 510(k) with new evidence, specifically as it relates to the subacute ...
Systemic toxicity tests include acute systemic toxicity tests and pyrogenic tests. ...
BySTEMart
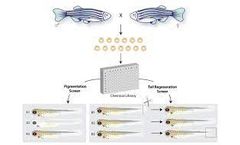
Backed by abundant experience and cutting-edge facilities, Creative Biostructure provides an ultimate MagHelix™ Zebrafish Screening Platform, which is designed to evaluate the activity and toxicity of compounds of drug development. There are four classes of zebrafish chemical screening assays: • Morphological screens (to screen chemical genetics and toxicology) ...
Creative BioMart Cosmetic Ingredients established by Creative BioMart to help scientists especially in the cosmetic fields, offers a wide range of cosmetics testing services to help customers meet the FDA requirements. The services mainly include: Raw Material Quality Assurance Test items include but are not limited to: Antibacterial agents and preservatives, ...
In addition to cell therapy, FUJIFILM Cellular Dynamics also offers life science research tools including the company’s inventoried iCell® products, which are available in almost any cell type and are sourced from multiple cell lines which can be applied for target identification as well as toxicity testing. The company also offers custom cell services ...
StemBioSys is further working to show the technology can improve preclinical drug/toxicity testing for other indications and cell types. ...
Food and Drug Administration (FDA), the CELLvo Matrix Plus technology is an accurate predictor of a drug's potential to cause cardiac toxicity. Many of paper's findings have been separately confirmed by StemBioSys' commercial partners, several of whom have validation studies underway. ...
The company operates worldwide from its 850m2 S1 facilities in Langenfeld, from where it also offers cell line engineering based on yeast (Hansenula polymorpha) and bacterial (E. coli) expression platforms, lab scale up- and downstream process development, supply of non-GMP bulk material (API) for activity and toxicity tests and technology transfer to cGMP ...
The method provides an alternative to three of the eleven current screening tests, most of which require animals. The Tox21 consortium is rapidly developing additional models to predict androgen and thyroid activity, which will provide alternatives for the other eight tests. ...
"We believe the results of pan-omic studies of drug induced liver damage will make a significant contribution to the understanding of metabolomic, proteomic and genetic associations and lead to more certain toxicity testing in multiple markets." About the Agilent Thought Leader Award The Agilent Thought Leader Award promotes fundamental scientific advances of ...
Environmental Protection Agency’s (EPA) ToxCast chemical screening program has awarded contracts to four United States-based companies to test up to 10,000 chemicals for potential toxicity to people and the environment. ...
Because Americans experience an astounding 52 million cases of the common cold each year (The Centers for Disease Control and Prevention), Seventh Generation, the nation's leading brand of environmentally responsible household and personal care products, and maker of a new, non-toxic, plant-derived Hand Wash, is encouraging Americans to take part in National Hand Washing ...
Staff from the National Institute of Environmental Health Sciences (NIEHS), one of the National Institutes of Health, and the National Toxicology Program (NTP) will speak at more than 30 different sessions and present 60 posters on topics ranging from improving toxicity testing to translational research. Many NIEHS grantees will also showcase their research. ...
With better understanding of how foods trigger allergic responses, scientists will be equipped to develop new tests for adverse effects from these foods and interpret data from toxicity tests required by regulation. ...