Refine by
Locations
- Africa
- Algeria
- Angola
- Benin
- Botswana
- Burkina Faso
- Burundi
- Cameroon
- Cape Verde
- Central African Republic
- Chad
- Comoros
- Congo
- Congo, Democratic Republic Of The
- Djibouti
- Egypt
- Equatorial Guinea
- Eritrea
- Ethiopia
- Gabon
- Gambia
- Ghana
- Guinea
- Guinea-Bissau
- Ivory Coast
- Kenya
- Lesotho
- Liberia
- Libya
- Madagascar
- Malawi
- Mali
- Mauritania
- Mauritius
- Morocco
- Mozambique
- Namibia
- Niger
- Nigeria
- Rwanda
- Senegal
- Seychelles
- Sierra Leone
- Somalia
- South Africa
- St. Helena
- Sudan
- Swaziland
- SãO Tomé & PríNcipe
- Tanzania
- Togo
- Tunisia
- Uganda
- Zaire
- Zambia
- Zimbabwe
Transcatheter Device Equipment & Supplies In Africa
6 equipment items found
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
In the early days of transcatheter mitral valve repair, the goal was a successful procedure that reduced mitral regurgitation (MR) to less than 3+; residual MR grade 2+ was considered acceptable.1,2 Compelling new data on more than 5400 patients show that residual MR grades 0–1+ are significantly associated with superior patient outcomes when compared with residual MR 2+, including ...
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
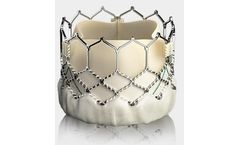
Built on the proven SAPIEN platform, the SAPIEN 3 Ultra transcatheter heart valve was designed with patient needs in ...
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
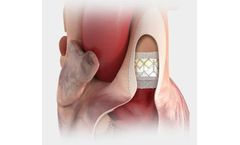
Designed for predictable deployment. The SAPIEN XT valve may be used in the aortic position via a broad range of access routes - transfemoral, transapical and ...
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
Builds upon the proven strengths of the SAPIEN valve with 5 years of durability. The SAPIEN 3 valve is the only valve approved for valve-in-valve procedures in both the aortic and mitral positions, allowing patients at high or greater surgical risk to avoid an additional open heart ...
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
The first transcatheter heart valve approved for pre-stented transannular patches. Also approved for use in the pulmonic position in conduits and ...
Manufactured by:Aran Biomedical Teoranta based inSpiddal, IRELAND
PTFE is a well-established biomaterial with a long clinical history of implantation. It has been approved by regulatory bodies for several decades and is used extensively in a range of vascular ...