Show results for
Refine by
Implant Guided Surgical Equipment & Supplies In Barbados
95 equipment items found
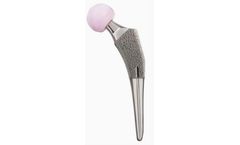
Manufactured by:Shoulder Innovations based inGrand Rapids, MICHIGAN (USA)
The InSet PLUS augmented glenoid offers surgeons the ability to dial the augment to the area of greatest defect. The InSet PLUS was designed to offer additional correction of the glenoid as it allows for the combination of eccentric reaming with an additional 5 & 10 degrees of correction. Like the standard InSet Glenoid, the InSet PLUS Augmented Glenoid has the same flat back design, which ...
Manufactured by:Nobel Biocare based inKloten, SWITZERLAND
A simply straightforward implant system. Exceptional versatility for universal use. Flexibility you'll appreciate whether you're an experienced clinician or new to implantology. Cover the majority of your cases with only one implant system – for implants in the anterior or posterior region and for treating single-tooth, multiple-teeth or full-arch ...
Manufactured by:NDI Medical based inBad Emstal, GERMANY
Designed for proper formation of the bone socket for the implant. Made of surgical stainless steel 1.4197 (X20 CrNiMoS13-1) that has been specially sharpened. Tensile strength Rm: 881-900 N/mm². Superior corrosion resistance and wear resistance. Depth mark grooves at 8, 10, 11.5, 13, and 15 mm. Additional laser marking inside the ...
Manufactured by:Exactech, Inc based inGainesville, FLORIDA (USA)
Design features to achieve immediate axial and rotational mechanical stability. Incorporates specific design features to achieve immediate axial and rotational mechanical stability between the lateral and medial cortices of the femoral ...
Manufactured by:International Polymer Engineering, Inc. (IPE) based inTempe, ARIZONA (USA)
IPE’s ePTFE suture components are soft, biologically inert and chemically non reactive. Our ePTFE suture components are ideal to limit inflammation, bleeding and other collateral effects which may occur during closure or soft tissue reconstruction. FluoroFlex™ ePTFE VS® suture components are manufactured in continuous lengths for suturing or custom applications. They are the ideal ...
Manufactured by:Matrix Surgical USA based inNewnan, GEORGIA (US) (USA)
Design Y Mandible Onlays benefit patients who have skeleton mandibular deficiencies or surgically altered anatomy. Implant design provides the opportunity for augmentation of ramus height and ramus width. It also provides the opportunity to alter the inclination of the mandibular plane and restore continuity of the mandibular border. The registration tab allows for ideal and symmetric placement. ...
Manufactured by:Restech based inHouston, TEXAS (USA)
Bridges the gap between conservative treatments and ...
Manufactured by:Sight Sciences, Inc. based inMenlo Park, CALIFORNIA (USA)
OMNI® Surgical System – an implant free device that targets outflow resistance. For surgeons, the OMNI® Surgical System is a novel, implant-free MIGS device which can be used in combination with cataract surgery or in a standalone procedure to lower IOP in adult patients with Primary Open Angle ...
Manufactured by:Corcym UK Limited based inLondon, UNITED KINGDOM
The Perceval Platform is based on a sutureless and collapsible design that simplifies the surgical implantation, reducing the impact of surgery and facilitating faster ...
Manufactured by:Anatomics Pty Ltd. based inMelbourne, AUSTRALIA
Polymer surgical implants are a permanent, predictable and symmetrical option for facial reconstruction or augmentation. StarPore® implants are an advanced polymer tissue scaffold indicated for the repair or augmentation of contours in the skull and face. Made from clinical proven porous polyethylene (HDPE), animal studies have shown StarPore® implants allow for rapid infiltration of ...
Manufactured by:GEUDER AG based inHeidelberg, GERMANY
Retinal implants are used in surgical treatment of retinal detachments. The implant creates an indentation in the sclera and choroid approximating the retina to the pigment epithelium. This procedure is accomplished by local indentation or through the use of an implant that encircles the ...
Manufactured by:Hi-Tec Implants based inHerzlya, ISRAEL
An internal hex tapered design implant system that is convenient to work with and which covers all bone conditions, with superior performance in dense cortical bone. ...
Manufactured by:Anatomics Pty Ltd. based inMelbourne, AUSTRALIA
Surgical Templates are designed by Anatomics engineers to assist the surgeon in confirming the fit of an Anatomics implant in certain cases. Existing tissue at the surgical site area may need modification to achieve optimal implant fit. A Surgical Template acts as a proxy for the implant to allow the surgeon to prepare the donor site appropriately. This reduces handling and potential ...
Manufactured by:Broncus Medical, Inc. based inSan Jose, CALIFORNIA (USA)
The InterVapor® System is a minimally invasive bronchoscopic treatment that is designed to deliver targeted Bronchoscopic Thermal Vapor Ablation (BTVA®) to ablate the most diseased hyper-inflated lung segments. The ablation results in a reduction in emphysematous tissue and volume in the targeted segment(s) allowing for healthy lung regions to expand and function more efficiently. BTVA is ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Non-bone tendons are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. Non-bone tendons used in ACL reconstruction procedures allow for multiple fixation ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Matrix HD Allograft Dermis is an acellular human dermis allograft sterilized to a Sterility Assurance Level (SAL) of 10-6 using the Tutoplast Tissue Sterilization Process. This proprietary process retains the three-dimensional intertwined multidirectional fibers and mechanical properties of the native dermis tissue. The Matrix HD graft provides a natural scaffold to support the body's ...
Manufactured by:W. L. Gore & Associates, Inc. based inNewark, DELAWARE (USA)
The GORE ACUSEAL Cardiovascular Patch is a high strength, low thrombotic, synthetic patch designed to decrease suture line bleeding in cardiovascular repair and reconstruction ...
Manufactured by:U.S. Titanium Industry Inc. (USTi) based inBaldwin Park, CALIFORNIA (USA)
Specification and Standard for Titanium Bar and Its Alloys: Apart from the products listed below, other available metal products are not listed here. Other metal products USTi can manufacture and supply include Tantalum, Niobium, Zirconium, Hafnium, Nickel, Copper etc., which are mainly in the form of Plate, Bar and ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI's Achilles tendons are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. These tendons allow for multiple techniques and fixation options in ligament reconstruction ...
Manufactured by:Zimmer Biomet CMF and Thoracic based inJacksonville, NEW JERSEY (USA)
Designed to conform to the contours of the sternum and to enhance approximation. Conforms more closely to the contours of the sternum than other less flexible wires. Meets the ASTM F138 & 5832-1 standards for implant grade surgical fixation sternotomy suture ...