Refine by
In Vitro Comparative Studies Equipment & Supplies In Belarus
83 equipment items found
Manufactured by:PharmAbcine Inc. based inYuseong-gu, SOUTH KOREA
PMC-402 is a monoclonal antibody that targets the human TIE2 that regulated activation, proliferation, and repolarization of Tie2 expressing macrophages, resulting in T cell activation. It binds to TIE2 expressing macrophages that activate T cells in the tumor microenvironment ...
Manufactured by:Futura Medical based inGuildford, UNITED KINGDOM
TIB200 is a topical 10% ibuprofen gel for pain relief that utilises Futura's DermaSys® technology. DermaSys® enables ibuprofen to be delivered rapidly and efficiently through the skin. Local application and rapid, efficient absorption translate into a fast onset of action, and the potential for twice-daily ...
Manufactured by:Futura Medical based inGuildford, UNITED KINGDOM
Scientific advisory meeting held with MHRA confirming the need of a Phase 3 study to support the improved skin permeation including potential superior efficacy claims. Exploring the feasibility of a clinical study to satisfy the Phase 3 requirements for both UK and US approval. Development currently on ...
by:Sumitomo Pharma Oncology, Inc. based inCambridge, MASSACHUSETTS (USA)
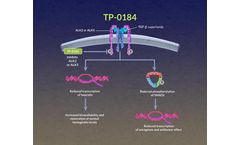
TP-0184 is an investigational inhibitor of activin receptor-like kinase 2 (ALK2) and ALK5 (also known as TGFβR1). This bimodal inhibitor is believed to downregulate multiple TGF-β superfamily signaling pathways and has shown antitumor activity in several ...
Manufactured by:Scion BioMedical based inMiami, QUEBEC (CANADA)
Our biopolymer-based Clo-Sur P.A.D. promotes hemostasis through its innovative formulation, structure and positive ionic charge. When Clo-Sur P.A.D. is applied to a wound, its positive charge immediately begins working to rapidly control bleeding. In-vitro laboratory studies have shown this occurs at a faster rate than many competing ...
Manufactured by:CELLINK based inBoston, MASSACHUSETTS (USA)
CELLINK® PCL is a high-molecular weight (Mn 50,000) thermoplastic linear polyester derived from caprolactone monomer. This biodegradable polyester has a melting point of 60° C. PCL provides a reinforcing structure to load-bearing tissue constructs, including bone grafts and osteochondral plugs developed for in vitro and in vivo studies. It can also provide a temporary scaffold for ...
by:Joanneum Research Forschungsgesellschaft mbH based in
The evaluation of wound dressings and medical devices (e.g. skin substitutes) is of great importance to ensure their effectiveness and safety. We can also determine the "mode of action" of these materials. ...
Manufactured by:Nemera based inLa Verpillière, FRANCE
Off-the-shelf devices available for key identified nasal drugs. Accelerate your sprint to market with our Device Equivalence Program. Based on FDA requirements, we have established a Device Equivalence Program in order to accelerate time-to-market and achieve in-vitro bioequivalence requirements. Our main objective is to preselect and propose an appropriate delivery system per identified drug ...
Manufactured by:Regenity based inParamus, NEW JERSEY (USA)
A sponge-like onlay with a leak resistant top layer that provides effective protection against CSF leakage. When hydrated, this highly drapeable matrix is easily repositionable and conforms to the complex surfaces of the brain or spinal cord. The completely resorbable matrix does not adhere to surgical instruments or ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI Surgical offers the only meniscal allograft with both bone and meniscus sterilized through the BioCleanse® Tissue Sterilization Process. The BioCleanse processed meniscus graft provides a sterilized alternative to traditional aseptically processed meniscus allografts and sets the standard for safety and ...
Manufactured by:InStar Technologies a.s. based inLiberec, CZECH REPUBLIC
With our OTF InStrip: technology we are able to work with a wide range of physiologically active substances, the delivery of which we can modify according to the nature of substance and requirements of the partner. The technology gives the advantage to produce products for both human and veterinary ...
Manufactured by:Zinereo Pharma based inPontevedra, SPAIN
Bacterial vaginosis (bv) and vulvovaginal candidiasis (vvc) are the most prevalent causes of symptomatic vaginitis1 lactobacilli represent almost 90% of vaginal microbiota of healthy women. Vaginal infections are characterized by a significant decrease in vaginal lactobacilli, which are associa-ted with an overgrowth of pathogens such as candida spp2.several studies revealed ...
Manufactured by:Creative Bioarray based inShirley, NEW YORK (USA)
Smooth muscle is responsible for the contractility of hollow organs, such as blood vessels, the gastrointestinal tract, the bladder, and the uterus. Its structure differs greatly from that of skeletal muscle. The human esophagus contains three layers of muscle in its walls, the outer longitudinal and inner circular layers of the main muscular coat and the muscular layer of the mucosa. Visceral ...
Manufactured by:InStar Technologies a.s. based inLiberec, CZECH REPUBLIC
Cebedix & Cebidix-h are an Oral Thin Films using our InStripTM technology with CBD (Canabidiol). InStrip technology allows you to use the full potential of the substances contained in Cebedix, in this case CBD. You will achieve the desired effect from a much smaller dose. And a smaller dose means a smaller toll on the organism. The first effects of Cebedix in the form of a thin, ...
Manufactured by:Garwood Medical Devices, LLC based inBuffalo, NEW YORK (USA)
Currently under investigation to study the elimination of biofilm infections on prosthetic knee implants during early intervention procedures while maintaining the current standard of ...
Manufactured by:Yeasen Biotechnology (Shanghai) Co.,Ltd. based inShanghai, CHINA
D-luciferin is a common substrate for Luciferase and is widely used throughout biotechnology, especially in vivo imaging Technology. The mechanism of action is that luciferin (substrate) can be oxidized to emit light in the presence of ATP and luciferase. When fluorescein is in excess, the quantum number of light produced is equal to Luciferase concentrations were positively correlated (see ...
Manufactured by:Gamma-Service Medical GmbH based inLeipzig, GERMANY
GSR C1 - Gamma irradiation of blood and blood derivatives, cell cultures, transplants for comfortable, effective and precise irradiation in transfusion medicine and research (3.8 liter irradiation ...
Manufactured by:Epithelix based inPlan-les-Ouates, SWITZERLAND
Epithelix is offering primary Human Alveolar Pneumocytes, low passage, isolated from biopsies. Alveolar pneumocytes are commonly used for in vitro studies related with pulmonary diseases and chemical ...
Manufactured by:CONMED Corporation based inLargo, FLORIDA (USA)
With it's small size and innovative instrumentation, the Y-Knot® Flex System was designed to help achieve optimal anchor placement. The anchors provide up to 55% greater fixation strength than conventional 3.0mm press fit anchors while removing up to 80% less bone.1 The curved and percutaneous delivery systems enable delivery around a curve and perpendicular placement for posterior SLAP and ...
Manufactured by:Aedicell, Inc. based inRio Grande, NEW JERSEY (USA)
The only product on the market that combines human tissue sourced from the amniotic/chorionic membrane, umbilical cord AND placental disc —Dermavest®. This combination delivers a myriad of cell attachment proteins (CAP) including collagen, proteoglycans, polysaccharides and cytokine/growth factors (GF’s) that, combined with the structural aspects of the placental connective tissue ...