Show results for
Refine by
Spine Fusion Equipment & Supplies In Cambodia
49 equipment items found
Manufactured by:Bonegraft Biyolojik Malzemeler San. VE TIC. A.S. based inIZMIR, TURKEY
Powerbone flexible strip is a bioresorbable synthetic bone graft that provides great hanling with high elasticity for specific cases including bone defects in the pelvis, extremities, and the posterolateral spine fusion. Powerbone flexible strip is composed of silicate additive β-TCP and PLA based synthetic polymer. Instructions of Implanting Powerbone ...
Manufactured by:Nexxt Spine, LLC based inNoblesville, INDIANA (USA)
The Blade® Anterior Cervical Plate System is intended for anterior Screw fixation during the development of cervical spine fusion. The spinal fixation device includes Self-Drilling/Self-Tapping Screws, patented ‘Hurricane’ locking mechanism, and pre-contoured 1-5 level plates. Various instruments are also available as part of the Blade® ...
Manufactured by:Griportho Surgicals Pvt Ltd based inVadodara, INDIA
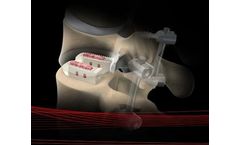
Size: 8.0 x 20 & 25mm, 9.0 x 20 & 25mm, 10.0 x 20 & 25mm, 11.0 x 20 & 25mm, 12.0 x 20 & 25mm, 13.0 x 20 & 25mm and 14.0 x 20 & 25mm. Indication: Achieves spinal fusion in the low back by inserting a cage directly into the disc space. Bone graft is placed in the cage and along the sides of the spine. Instrument system: PLIF/TLIF Cage ...
Manufactured by:Ulrich GmbH & Co. KG based inUlm, GERMANY
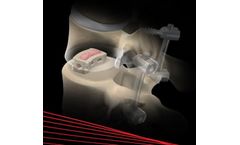
System: Dorsal dynamic stabilization, from the thoracic to the sacral spine. Non-fusion approach. Screw with hinged joint. Cannulation and self-cutting thread. Bonit® coating. Sterile packed ...
Manufactured by:Griportho Surgicals Pvt Ltd based inVadodara, INDIA
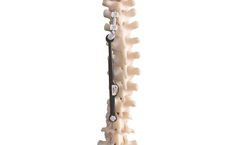
Raw Material: Titanium Grade – 5. Diameter: Ø 4.5, 5.5, 6.5 and 7.0mm. Length: 25, 30, 35, 40, 45, 50 and 55mm. Indication: Act as a firm anchor points that can then be connected with a rod creating a construct that prevents motion during Spinal Fusion ...
Manufactured by:Xtant Medical based inBelgrade, MONTANA (USA)
Matriform Si was developed to resemble the composition and porous structure of natural human bone. Comprised of 96% pure phase β-TCP granules, 4% silicate and collagen, Matriform Si provides the ideal biomimetic scaffold for spinal fusion procedures. The flexible strip offers excellent handling and shape memory ensuring direct contact with the surface of healthy ...
Manufactured by:Orthomerica Products, Inc. based inOrlando, FLORIDA (USA)
The California Catalina Orthosis is easy-to-fit and simple for the patient to don and doff. The orthosis limits motion and provides effective compression for the relief of lower back pain — making it ideal throughout rehabilitation. The Catalina 631 and 637 both deliver effective compression to the region of pain localizing the patient’s therapy while maintaining comfort for the ...
Manufactured by:OrthoPediatrics Corp. based inWarsaw, INDIANA (USA)
Designed to fill a crucial gap in the treatment of adolescent idiopathic scoliosis (AIS), the ApiFix procedure provides a viable alternative to both bracing and fusion for select patients. Less Invasive: With three pedicle screws and a familiar posterior approach, the unilateral technique is less invasive than spinal fusion and simpler than the other fusionless procedures requiring an anterior ...
Manufactured by:Shanghai Kinetic Medical Limited Co., Ltd. based inShanghai, CHINA
Cerven™ Anterior Cervical Plate System. Low profile curved edge. Reduce tissue irritation. Unique locking mechanism. Anti-override design. 90° clockwise locking. Pre-bent design. Fit physiological ...
Manufactured by:Anatomics Pty Ltd. based inMelbourne, AUSTRALIA
SpineBox™ is an innovative, customised kit that uses individual patient CT scan data to create patient specific devices and pre-plan the selection of spinal fusion hardware in both open and minimally invasive spinal surgery. In minimally invasive approaches (such as TLIF) each SpineBox™ can include pre-selected standard fusion hardware with a patient ...
Manufactured by:Xtant Medical based inBelgrade, MONTANA (USA)
A standalone low profile anterior cervical integrated fusion system featuring titanium plasma-coated teeth and two locking screws protected by a resilient locking arm mechanism. The Irix-C Cervical Integrated Fusion System consists of an integrated endoskeleton, surrounded by an outer PEEK ring and two screws. It is intended for spinal fusion procedures at one level (C3–T1 inclusive) in ...
Manufactured by:MedSpark, LLC based inSan Luis Obispo, CALIFORNIA (USA)
The pedicle screw, which is sometimes used as an adjunct to spinal fusion surgery, provides a means of gripping a spinal segment. The screws themselves do not fixate the spinal segment, but act as firm anchor points that can then be connected with a rod. The screws are placed by the surgeon into two or three consecutive spine segments (e.g. lumbar segment 3 and ...
Manufactured by:Anatomics Pty Ltd. based inMelbourne, AUSTRALIA
Patient specific titanium spine cages and plates can be fabricated individually from CT data to allow surgeons to restore the natural alignment of the spine and assist fusion in cases where a standard off-the-shelf implant is not suitable. These implants can be designed for either anterior or posterior fusion or vertebral ...
Manufactured by:ApiFix Ltd. based inYokneam Illit, ISRAEL
The ApiFix procedure offers an innovative surgical option for correction of progressive adolescent idiopathic scoliosis (AIS). It fills a crucial treatment gap, offering a less invasive, motion-preserving option for curve correction – without the permanence of ...
Manufactured by:ARCH Medical Solutions Corp. based inScarborough, MAINE (USA)
From cervical to lumbar to SI fusion, we develop spinal devices used to treat deformity, stabilize and strengthen the spine while facilitating fusion. Devices can now be optimized to better match the modulus of bone and also provide better surgical visibility through the use of biocompatible titanium. Additive manufacturing is revolutionizing ...
Manufactured by:Alliance Spine based inSan Antonio, TEXAS (USA)
Transforaminal lumbar intervertebral device manufactured from PEEK Optima LT1 with tantalum markers for radiographic ...
Manufactured by:Leader Biomedical Group based inAmsterdam, NETHERLANDS
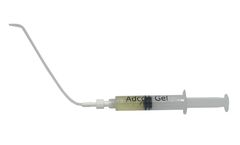
Adcon® Gel is a biocompatible, resorbable gel that provides a physical barrier to intertissue adhesions and inhibits fibroblast migration. This results in improved patient rehabilitation, reduced recurrent pain, and decreased number of re-operations as a result of such ...
Manufactured by:icotec ag based inAltstätten, SWITZERLAND
Integrated with the Spine; The best of both worlds: postoperative visibility and secure fixation to bone. The BlackArmor® material is radiolucent and integrates with the vertebral endplates thanks to the rough Ti-iT® titanium ...
Manufactured by:Alliance Spine based inSan Antonio, TEXAS (USA)
Posterior lumbar intervertebral device manufactured from PEEK Optima® LT1 with tantalum markers for radiographic ...
Manufactured by:Xtant Medical based inBelgrade, MONTANA (USA)
OsteoSelect was engineered for superior performance. The graft does not adhere to gloves yet maintains its placement in the surgical environment. Osteoinductivity of sterile final product is assessed in vivo. In this challenging model, every lot tested to date has consistently demonstrated an osteoinductive response. This performance validates Xtant Medical’s expertise in DBM processing. ...