Show results for
Refine by
Intervertebral Disc Equipment & Supplies In Canada
24 equipment items found
Manufactured by:DiscGenics, Inc. based inSalt Lake City, UTAH (USA)
DiscGenics is harnessing the restorative potential of cells native to the intervertebral disc to develop what we believe will be a profound therapeutic option for millions of patients suffering from the debilitating effects of low back ...
Manufactured by:Neo Medical Sa based inVillette, SWITZERLAND
Degenerative scoliosis, describes a side-to-side curvature of the spine caused by degeneration of the facet joints and intervertebral discs which are the moving parts of the spine. This adult degeneration and resulting spinal asymmetry can occur slowly over time as a person ages, and may cause symptoms including lower back and/or leg pain. ...
Manufactured by:DiscGenics, Inc. based inSalt Lake City, UTAH (USA)
DiscGenics uses its patented allogeneic cell culture manufacturing technology to isolate cells from donated adult human intervertebral disc tissue and induce a progenitor state. These proprietary Discogenic Cells are then expanded for therapeutic application, combined with a delivery vehicle and cryopreserved for off-the-shelf ...
Manufactured by:8sense GmbH i. L. based inRosenheim, GERMANY
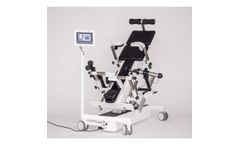
Happens almost incidentally there, where you spent the most time of your day. 12h sitting per day. One-sided strain for your muscles. Decreasing productivity. Adverse effects on your intervertebral discs. The consequences of one-sided sitting at work are tremendous and cannot be fixed with a simple after-work training. ...
Manufactured by:BPB MEDICA based inMirandola (MO), ITALY
A hernia is a common disorder that occurs when the intervertebral discs nucleus pulposus becomes dislodged from its natural position inside the annulus fibrosus. Most annulus fibrosus lesions are the result of repeated microtraumas or a major trauma that degenerates to allow the nucleus pulposus to bulge and compress the spinal cord or a nerve, causing pain in ...
Manufactured by:Fysiomed CS based inTrinec, CZECH REPUBLIC
The treatment regimen for scoliosis consists of two to three sessions per week, each involving two 20-minute therapy intervals. Other conditions, like the inflammation of intervertebral discs and vertebrae, require more frequent sessions, three to four times weekly. GraviSpine's structured approach offers distinct therapeutic benefits aimed at improving spine ...
Manufactured by:Centinel Spine based inWest Chester, PENNSYLVANIA (USA)
The most studied and clinically-proven total disc replacement technology in the world. Determined Safe & Effective for SCDD: The prodisc C Nova Total Disc Replacement is intended to replace a diseased and/or degenerated intervertebral disc of the cervical spine in patients with symptomatic cervical disc ...
Manufactured by:GELITA AG based inEberbach, GERMANY
Collagen is crucial for mobile joints, stable bones, healthy muscles, strong ligaments and tendons, smooth skin, glossy hair and healthy finger nails. It is one of the primary structural proteins of connective tissues and also abundant in blood vessels, intervertebral discs, the blood-brain barrier, the cornea, dentin and the intestinal wall – a vital ...
Manufactured by:Wuhan Gigaa Optronics Technology Co.,Ltd. based inWuhan, CHINA
Whether it is for general vet practice, specialist veterinary centres, physiotherapy and rehabilitation specialists, or universities, we have a Diode laser for you. By easily changing hand pieces you will be able to perform therapy, surgery, dentistry, percutaneous intervertebral disc decompression, tooth whitening, and ...
Manufactured by:Spineway S.A based inÉcully, FRANCE
Spineway specializes in the development and production of advanced disc prostheses designed to preserve natural spinal motion in patients undergoing surgical interventions for degenerative spinal conditions. Motion preservation technologies offer an alternative to spinal fusion by maintaining the mobility of the spine post-surgery, thus improving patient outcomes. These ...
Manufactured by:Spineway S.A based inÉcully, FRANCE
Their products feature a range of cervical and lumbar cages specifically designed for ACIF and TLIF procedures. The interbody fusion process involves the use of implants, known as interbody cages, to replace damaged intervertebral discs, aiding in the restoration of disc height and ensuring proper spinal alignment. These cages also promote bone ...
Manufactured by:Miracle Orthopedics based inCairo, EGYPT
Reinforced by suitable metal or semirigid strips incorporated posteriorly on each side of sprinous processes to provid wide stablearea ...
Manufactured by:Radimed GmbH based inBochum, GERMANY
Laser fibers for PLDD; We offer you laser fibers and accessories for percutaneous laser disk decompression (PLDD) for different laser systems. The Radimed PLDD set includes a laser fiber, a fiber fixation adapter and a scaled special cannula with a diameter of 18G or 21G. The fiber fixation enables a precise connection of the fiber to the cannula and prevents the laser fiber from slipping into ...
Manufactured by:Medicrea based inRillieux-la-Pape, FRANCE
Enhanced stability: the anatomical dome design with a central wall of IMPIX-C™ provides an excellent stability especially in rotation and offers an efficient fusion solution. Optimum fusion: thanks to 2 windows for graft ...
Manufactured by:Medicrea based inRillieux-la-Pape, FRANCE
Sterile PEEK Optima Cervical Interbody Device, pre-assembled on holder/impactor, and delivered with disposable instrumentation ...
Manufactured by:Lasotronix Sp. z o.o. based inPiaseczno, POLAND
Percutaneous laser disc decompression (PLDD) is a minimally invasive procedure that aims to significantly reduce the patient’s pain and reduce the neurological ...
Manufactured by:3D Medical based inMarolles-en-Brie, FRANCE
Used during horses cervical arthrodesis in cases of fracture, dislocation and vertebral instability or misalignment (cervical stenotic ...
Manufactured by:Excite Medical Corp. based inTampa, FLORIDA (USA)
The DRX9000C® can be added to any existing DRX9000® True Non-Surgical Spinal Decompression System or purchased as a combination system. The DRX9000C® relieves patients’ neck (cervical) pain through a detachable ...
Manufactured by:Technomex based inGliwice, POLAND
Modern traction table for cervical and lumbar spine with three dimensional correction arrangement of laying surface using precise gas springs. Platinum allows to perform static, harmonic and intermittent traction. Touch screen panel greatly simplifies treatment process and patient database allows quick access to information about the progress in therapy and used treatment ...
Manufactured by:Hoogland Spine Products GmbH based inFrankfurt am Main, GERMANY
MaxDisc provides treatment for patients who have failed conservative care and are not yet ready for major surgery. It is a minimal access procedure performed on an outpatient basis. Following manual removal of the offending herniation, the MaxDisc ® device is activated to help clean the disc and seal tears in the annulus. As a result, pressure in the disc is reduced, which eases ...