Show results for
Refine by
Fusion Surgery Equipment & Supplies In Croatia
17 equipment items found
Manufactured by:Griportho Surgicals Pvt Ltd based inVadodara, INDIA
Indication: Act as a firm anchor points that can then be connected with a rod creating a construct that prevents motion during Spinal Fusion ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
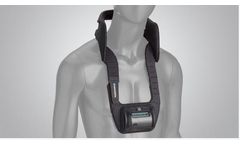
The SpinalStim device is FDA approved to be used after spinal fusion surgery or to be used to treat a failed fusion from a previous surgery. The devices stimulate the natural healing process of bone by sending low-level pulses of electromagnetic energy to the injury or fusion site. The device has an overall ...
Manufactured by:AF Medical GmbH based inSpaichingen, GERMANY
Highest quality spine implants consisting of instruments and implants for the most common applications in cervical, lumbar and thoracic fusion surgery. ...
Manufactured by:MedSpark, LLC based inSan Luis Obispo, CALIFORNIA (USA)
The pedicle screw, which is sometimes used as an adjunct to spinal fusion surgery, provides a means of gripping a spinal segment. The screws themselves do not fixate the spinal segment, but act as firm anchor points that can then be connected with a rod. The screws are placed by the surgeon into two or three consecutive spine segments (e.g. lumbar segment 3 and ...
Manufactured by:HCM Orthocare Pvt. Ltd. based inAhmedabad, INDIA
HCM Orthocare specializes in manufacturing high-quality cervical plates utilized in spinal surgery, focusing on ensuring the stability and support of the cervical spine after injuries, fractures, or fusion surgeries. These plates are crafted from premium materials like titanium and stainless steel, offering superior strength, durability, and ...
Manufactured by:Cerebro Spine based inIzmir, TURKEY
Anterior Cervical disc prosthesis is made of titanium alloy (Ti6Al4V) -ELI suitable for imaging techniques such as MRI-CT produced for in-body applications and MR compatible peek material (EVONIK), which is a body compatible thermoplastic solution for in-vivo applications and does not create any ...
Manufactured by:Kuros Biosciences A.G. based inSchlieren, SWITZERLAND
MagnetOs isn’t like other bone grafts. It grows bone even in soft tissue thanks to our unique NeedleGrip™ surface technology which provides traction for our body’s vitally important ‘pro-healing’ immune cells (M2 macrophages).*†6,7 This in turn, unlocks previously untapped potential to stimulate stem cells – and form new bone throughout the graft.*8-10 ...
Manufactured by:Advin Urology based inAhmedabad, INDIA
ADVIN Vessel Sealing System (SAFESEAL +) is a next-generation in energy-based vessel sealing technology. It presents an improved and safe vessel sealing procedure in the medical sector. It is known as Vessel Sealing Machine, Vessel Sealing Diathermy Unit, Monoseal Vessel Sealing System, High-Frequency Diathermy Machine With Vessel Sealing Unit, Laparoscopic Vessel Sealing System And Vessel ...
Manufactured by:Shanghai Kinetic Medical Limited Co., Ltd. based inShanghai, CHINA
The V-Crest is a titanium interspinous insert system with an oval liner (central axis), tissue expander and retaining wings on both sides, of which the liner material is PEEK. The product is used to open up the lumbar spinal space and relieve symptoms of lower back pain caused by discogenic disease or stenosis of the spinal canal or vertebral ...
Manufactured by:MedSpark, LLC based inSan Luis Obispo, CALIFORNIA (USA)
Cervical spinal fusion (arthrodesis) is a surgery that joins selected bones in the neck (cervical spine). Cervical fusion is sometimes used to treat acute injuries, such as fractures and dislocations, and to treat mechanical or structural problems causing disabling pain (such as from cancer or infection). In many instances, cervical ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The PerQdisc is designed for use using a lateral traspsoas or retroperitoneal (i.e., anterolateral) surgical approach to access disc space. Access through the annulus can be achieved using the PerQdisc™ Disc Access System (Figure 3). Once annular access is achieved, a nuclectomy (removal of nucleus material) is performed through the disc access using various tools to remove most of the ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
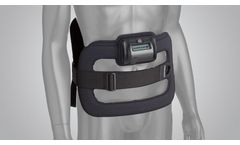
The CervicalStim device is the only bone growth stimulation therapy approved by the FDA as a noninvasive, adjunctive treatment option for cervical fusion in patients at high-risk for non-fusion. The device uses a pulsed electromagnetic field (PEMF) to induce a low-level electrical field at the fusion site which stimulates bone healing at a ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The PerQdisc Implant Delivery Device (Figure 4) is then inserted through the Access Cannula into the enucleated disc space. Contrast is injected into the inner chamber of the implant. This aids in visualization as well as provides real-time monitoring of fill pressure. The outer chamber of the implant is then filled under pressure control with a curable, polymer containing barium sulfate. The ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The SST PerQdisc Nucleus Replacement System is intended to replace the nucleus pulposus of the intervertebral disc in the L1-S1 spinal region in patients with single-level discogenic pain. The patient may have single or multi-level degenerative disc changes but the discogenic pain must be limited to a single level. The patient must be skeletally mature, at least 21 years of age, presenting with ...
Manufactured by:Hoogland Spine Products GmbH based inFrankfurt am Main, GERMANY
Biportal Endoscopic Spine System, BESS, comes out to be one of new trends of treatment of degenerative spine disorders. Literally it has a deeper origin from bilaterally (bilateral approach, one(left) side for scoping and the other(right side) for working) biportal arthroscopic approach by Dr. Schubert and Hoogland1), ...
Manufactured by:Teknimed based inL’union, FRANCE
Thanks to its composition, NANOGEL® is a synthetic bone substitute similar to mineral phase of bone. This product is an absorbable bone void filler which induces a rapid bone ingrowth. NANOGEL® offers to surgeon various possibilities of use : percutaneous surgery to fill closed bone defects or open ...
Manufactured by:Bonetech Medisys based inAhmedabad, INDIA
The Magnifica™ FNC Pedicle Screw offers enhanced fixation through the innovative use of bone cement injection directly into the vertebral body upon screw placement. This technique aims to address the challenges of achieving adequate stabilization, particularly in patients with osteoporotic ...