Show results for
Refine by
Blood Vessel Damage Equipment & Supplies In Guam
8 equipment items found
Manufactured by:I-Tech Medical Division based inMartellago (VE), ITALY
I-Press is a home pressotherapy device that is ideal for the treatment of diseases affecting the circulatory system, in order to improve the peripheral blood ...
Manufactured by:Purple Surgical based inShenley, UNITED KINGDOM
The Purple Surgical Ultimate Hasson Trocar Range is designed for surgeons who prefer the security of the open, cut down technique for the primary ...
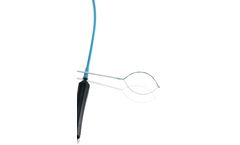
Manufactured by:endox Feinwerktechnik GmbH based inDettingen/Erms, GERMANY
With the en-bloc removal of polyps or lesions the speed of the cut is vital to ensure the blood vessels are successfully sealed. The slower the cut is made, the more effective it is. However, if it is done too slowly, the organ wall can be thermally damaged. The ENDOx polypectomy snare takes a new approach. One half of the symmetrically opening ...
Manufactured by:Roche Diagnostics International AG based inRotkreuz, SWITZERLAND
The cobas® Factor II and Factor V Test is an in vitro diagnostic device that uses real-time Polymerase Chain Reaction (PCR) for the detection and genotyping of the human Factor II (Prothrombin) G20210A mutation and the human Factor V Leiden G1691A mutation, from genomic DNA obtained from K2EDTA whole blood specimens, as an aid in diagnosis of patients with suspected thrombophilia. The ...
Manufactured by:Longest - Guangzhou Longest Science & Technology Co., Ltd. based inGuangzhou, CHINA
LGT-2200WM is a compression therapy device comprising an intermittent pneumatic controller, 8-chamber sleeves and a connectable hose. The controller inflates air into the sleeves, forms gradient pressures, and performs inflation and deflation. The inflate-deflate process pushes the accumulated venous blood and lymph fluid back to the effective blood circulation, with directivity, graduality, and ...
Manufactured by:U.S. Stem Cell, Inc. (USRM) based inSunrise, FLORIDA (USA)
MyoCell SDF-1 has received approval from the FDA to begin human clinical trials and is intended to be an improvement to MyoCell. The myoblast cells to be injected are gene-modified prior to injection by an adenovirus vector so that they will release extra quantities of stromal derived factor – 1 or SDF-1 protein. Following injury which results in inadequate blood flow to the heart, such as ...
Manufactured by:Tennessee Medical Innovations Inc., a part of the Mundomedis Group. based inClarksville, TENNESSEE (USA)
The SmartVision Arthroscopy Pump, developed by Insightra, is an advanced technology designed to enhance surgical procedures through efficient fluid management. The pump features a patented technology that automatically adjusts fluid pressure and flow rates, improving the precision and effectiveness of arthroscopic surgeries. It integrates an optical detector located behind the Patient Cassette, ...
Manufactured by:Rumex International Co. based inTampa, FLORIDA (USA)
Surgery has progressed from simple wound debridement to repairs of the smallest tissues. Before, injured blood vessels and nerves can no longer be repaired. The only recourse was to cut the damaged blood vessel and cauterize the ends to prevent hemorrhage. Damaged nerves were left to either heal ...