Show results for
Refine by
Applications
- Microbiome Therapeutics for Clinical Trials
- Flagship Coil for Major Depressive Disorder (MDD) Treatment
- Flagship Coil for Anxious Depression Treatment
- Flagship Coil for Obsessive-Compulsive Disorder (OCD) Treatment
- Flagship Coil for Smoking Addiction Treatment
- Flagship Coil for Bipolar Disorder Treatment
- Flagship Coil for Post Traumatic Stress Disorder Treatment Treatment
- Flagship Coil for Schizophrenia (Negative Symptoms) Treatment
- Flagship Coil for Alzheimer’s Disease Treatment
- Flagship Coil for Autism Disorder Treatment Technology
Clinical Stage Equipment & Supplies In Iran
36 equipment items found
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
As part of its comprehensive Public Health and Biodefense vaccine franchise, under a license from the Henry M. Jackson Foundation, Auro Vaccines is developing a subunit vaccine (HeV-sG-V) composed of the envelope glycoprotein of Hendra virus for the prevention of Nipah virus (NiV) ...
by:NeoTherma Oncology based inWichita, KANSAS (USA)
NeoTherma Oncology is a clinical stage medical device company developing a proprietary Thermal Treatment (VectRx) system for deep solid tumors. Our technology is based on applying a safe, non-invasive, non-ionizing electromagnetic field to produce local-regional hyperthermic temperatures in tumor tissue, intended to increase the effectiveness of anticancer ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
Auro Vaccines has developed VesiculoVax™-vectored vaccines for pre- and post-exposure protection against the hemorrhagic disease caused by multiple species of filovirus (i.e. Zaire ebolavirus, Sudan ebolavirus and Marburg ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
Auro Vaccines is developing a GeneVax prime/VesiculoVax boost therapeutic HPV vaccine for use as an adjunct to surgery that targets the seven HPV types responsible for more than 92% of cervical and head and neck ...
Manufactured by:Elpiscience based inShanghai, CHINA
ES002’s dual ATP-adenosine mechanism is a potentially powerful TME regulator and transformative cancer immunotherapy. In preclinical studies ES002 demonstrated significant single agent anti-tumor activity as well as an excellent tolerability profile. ES002 has also shown superior enzymatic inhibition and binding ...
Manufactured by:Adrenomed AG based inBerlin, GERMANY
The Company’s lead product candidate is Adrecizumab (HAM8101; INN: enibarcimab), a clinical-stage, first-in-class drug targeting loss of vascular integrity. The strong rationale for Adrecizumab is supported by the elegance of its mode of action, a monoclonal antibody that on binding to its target Adrenomedullin preserves its functionality as regulator of ...
Manufactured by:Femasys Inc. based inSuwanee, GEORGIA (US) (USA)
FemBloc features our proprietary delivery platform, which places balloon technology close to the opening of both fallopian tubes. This in-office approach is designed to eliminate the risks of incisions, anesthesia, and hormones. As a nonsurgical procedure that is implant free, FemBloc delivers a biopolymer that is expected to expel within 3 months. Confirmation of procedure success can be ...
by:Verb Biotics based inBoston, MASSACHUSETTS (USA)
Yso1 is a clinical stage development program aiming at harnessing the potential of Christensenella minuta as a groundbreaking biotherapy to treating obesity and metabolic disorders. The goal of our disruptive approach is to return to obese patients christensenella, which have the unique ability to repair the unbalanced microbiome, thereby restoring impaired key ...
Manufactured by:AMS Biotechnology (Europe) Ltd. based inAbingdon, UNITED KINGDOM
Breast cancer with adjacent normal breast tissue array (2012 WHO classification), including pathology grade, TNM and clinical stage (AJCC 7th edition), 12 cases/24 cores, repalcing BR245. Breast carcinoma with adjacent normal breast tissue microarray, containing 10 cases of invasive carcinoma of no special type, 2 adjacent normal breast tissue, duplicate cores ...
Manufactured by:Navigo Proteins GmbH based inHalle, GERMANY
Navigo Proteins’ Precision Capturing® technology dramatically simplifies non-Fc biologics downstream processing from pre-clinical stage to commercial manufacturing. This is achieved by substituting several chromatography steps by one single custom affinity chromatography capture step based on Navigo’s proprietary artificial Protein A affinity ...
Manufactured by:Frontier Biotechnologies Inc. based inNanjing, CHINA
Licensed with exclusive rights for greater China from AFFiRiS AG, a clinical-stage biotechnology company in Austria, FB6001 is an investigational therapeutic peptide vaccine targeting PCSK9 to treat hypercholesterolemia. PCSK9 is a clinically validated target and FB6001 is a potential first-in-class immunotherapy for hypercholesteremia. FB6001 is ...
Manufactured by:Vivesto AB based inSolna, SWEDEN
Cantrixil is a product candidate in clinical stage being developed for the treatment of ovarian cancer. Cantrixil consists of the active molecule, a potent and selective third generation benzopyran SMETI inhibitor named TRXE-002-01, encapsulated in a cyclodextrin. It is believed to target a wide spectrum of cancer cells, including chemotherapy-resistant ...
Manufactured by:Applied Pharma Research s.a. based inBalerna, SWITZERLAND
APR TD012 is a clinical stage drug candidate for the treatment of wounds in Hailey Hailey Disease (HHD) patients. The product improves healing process thanks to its bioburden control and its anti-oxidant properties.Oxidative stress has been demonstrated to play a specific role in the pathogenesis of HHD by regulating the expression of factors playing an ...
Manufactured by:Vaxcyte, Inc. based inSan Carlos, CALIFORNIA (USA)
We believe our clinical-stage company is uniquely positioned to create vaccines that can overcome bacteria’s formidable defense mechanisms and be produced on a significant scale. We are leveraging our XpressCF™ cell-free protein synthesis platform to create high-fidelity vaccines featuring distinct protein carriers and antigens – the ...
Manufactured by:Brainlab AG based inMunich, GERMANY
This pre-planning and planning workflow, comprised of an array of highly-automated software applications, addresses specific clinical challenges at each stage of the planning process. The Multiple Brain Mets Workflow allows you to create single isocenter plans in minutes, guaranteeing target coverage while offering excellent conformity and steep dose ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
The Auro Vaccines discovery engine is rapidly creating a pipeline of differentiated new vaccines. On the leading edge of development ...
Manufactured by:Rocket Pharmaceuticals, Inc. based inCranbury, NEW JERSEY (USA)
The LVV platform is ideal for modifying hematopoietic stem cells (HSCs) to address disorders affecting the bone marrow. The LVV transduction process occurs ex vivo (outside the body), which ensures that the gene has been properly integrated before the therapy is given to the patient. The process involves collection and isolation of a patient’s hematopoietic stem cells ...
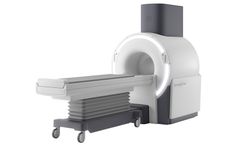
Manufactured by:Synaptive Medical based inToronto, ONTARIO (CANADA)
Bring MRI closer to the patient to support faster and earlier detection of neurological deficits. In situations where every second counts, the Synaptive MRI holds the potential to bring MRI closer to the patient to support faster and earlier detection of neurological deficits. The overarching challenge for wider utilization of MRI has been accessibility in both critical and acute situations. The ...
Manufactured by:Rocket Pharmaceuticals, Inc. based inCranbury, NEW JERSEY (USA)
The LVV platform is ideal for modifying hematopoietic stem cells (HSCs) to address disorders affecting the bone marrow. The LVV transduction process occurs ex vivo (outside the body), which ensures that the gene has been properly integrated before the therapy is given to the patient. The process involves collection and isolation of a patient’s hematopoietic stem cells ...
Manufactured by:OPKO Health, Inc. based inMiami, FLORIDA (USA)
Rayaldee® (calcifediol) is an extended-release prohormone of the active form of vitamin D3 that both raises 25-hydroxyvitamin D and lowers intact parathyroid hormone (iPTH) levels in patients with stage 3 or 4 CKD. Rayaldee® is under development for adult patients with stage 5 chronic kidney disease (CKD) with SHPT and vitamin D insufficiency who require regular ...