Show results for
Refine by
Coronary Artery Equipment & Supplies In Lebanon
119 equipment items found
Manufactured by:Circle Cardiovascular Imaging Inc. based inCalgary, ALBERTA (CANADA)
Specialized tools for the assessment of coronary artery disease using cardiac CT. Read and report Cardiac CT with zero-click coronary artery segmentation and quantifying calcium scoring powered by ...
Manufactured by:United Biologics, Inc. based inIrvine, CALIFORNIA (USA)
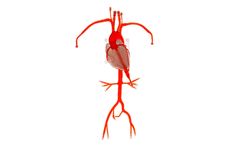
The Embedded Coronary Model is our three-dimensional platform for the left and right coronary artery systems. This transparent, compliant model features the major coronary arteries embedded on the contours of a human heart base. This vessel also features femoral access, subclavians, aorta, renals and iliofemoral ...
Manufactured by:ASAHI INTECC CO., LTD. based inAichi, JAPAN
Tubes inserted into blood vessels from the point between the puncture site and the entrance of the coronary artery. Safely navigates medical devices such as PTCA guiding wires and PTCA guiding catheters to the entrance of coronary ...
Manufactured by:Biosensors International Group, Ltd. based inSingapore, SINGAPORE
The use of the BioStream PTCA catheter is indicated for patients with discrete lesions of the coronary arteries with a reference vessel diameter of 1.50 to 4.00 mm for the purpose of improving the coronary lumen diameter. The BioStream PTCA catheter is indicated for the dilatation of the affected segments of a coronary ...
Manufactured by:Wellinq based inLeek, NETHERLANDS
High quality Angiographic catheter that provides reliable kink-free passage of tortuous anatomy and calcified lesions while keeping optimal torque control in the coronary ...
Manufactured by:Creative Peptides based inNew York, NEW YORK (USA)
Perindopril Erbumine is the stable form of perindopril, which is a long-acting ACE inhibitor used to treat high blood pressure, heart failure, or stable coronary artery disease.http://www.creative-peptides.com/product/perindopril-erbumine-item-10-101-120-88.html ...
Manufactured by:SOFIE based inDulles, VIRGINIA (USA)
N13-Ammonia is a radioactive diagnostic agent for Positron Emission Tomography (PET) indicated for diagnostic PET imaging of the myocardium under rest or pharmacologic stress conditions to evaluate myocardial perfusion in patients with suspected or existing coronary artery ...
Manufactured by:OpSens Medical, a division of OpSens based in, QUEBEC (CANADA)
OpSens OptoWire is a modern pressure guidewire designed to assess stenoses in vessels such as coronary arteries. OptoWire is powered by Fidela™, a patented 2nd generation fiber optic sensor to measure physiologic indices including Fractional Flow Reserve (FFR and diastolic Pressure Ratio (dPR). ...
Manufactured by:Bioprojet based inParis, FRANCE
Kengrexal, co-administered with acetylsalicylic acid (ASA), is indicated for the reduction of thrombotic cardiovascular events in adult patients with coronary artery disease having undergone a percutaneous coronary intervention (PCI), who have not received an oral P2Y12 receptor inhibitor prior to the intervention and in whom the oral ...
Manufactured by:Concept Medical Inc. based inTampa, FLORIDA (USA)
Novel, First of its kind Sirolimus drug coated balloon catheter for the treatment coronary artery disease. MagicTouch is intended for in-stent restenosis, small vessels, bifurcation lesions and de-novo lesions ...
Manufactured by:Invamed based inChampionsGate, FLORIDA (USA)
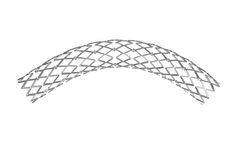
Atlas? is a coronary balloon-expandable Stainless Steel stent system consisting of everolimus particles. It is indicated for de novo and in-stent restenotic lesions in patients with coronary artery disease – including acute coronary syndrome acute myocardial infarction or unstable angina and/or concomitant diabetes mellitus ...
Manufactured by:Elixir Medical Corporation based inMilpitas, CALIFORNIA (USA)
The DynamX Drug Eluting Coronary Bioadaptor System is a significant innovation in the treatment of coronary artery disease. Going beyond drug-eluting stents (DES), DynamX represents one of the most significant breakthroughs in implant design in the past 30 ...
Manufactured by:CARDIONOVUM GmbH based inBonn, GERMANY
Unmatched XLIMUS clinical performance. The ultimate DES for complex artery lesions. XLIMUS is considered as the ultimate coronary DES stent system to treat complex coronary artery disease by reaching and crossing the most challenging ...
Manufactured by:Gadelius Medical K.K. based inTokyo, JAPAN
This thrombus-removal catheter allows for selective imaging of the coronary arteries, and the infusion of drugs such as thrombolytic agents, with the guide wire left in place. It has a Rapid Exchange type catheter structure, making use of a soft tip with high guide wire followability. ...
Manufactured by:Celixir plc based inStratford-upon-Avon, UNITED KINGDOM
PHASE 2 : Our first investigational cardiac regenerative medicine consists of iMP cells. iMP cells are designed to treat patients with ischaemic heart disease – ischaemic cardiomyopathy – undergoing coronary artery bypass graft (CABG). They are injected around cardiac scar tissue via a single application during bypass ...
Manufactured by:Invamed based inChampionsGate, FLORIDA (USA)
Atlas is a coronary balloon-expandable CoCr (L605) stent system consisting of everolimus particles. It is indicated for de novo and in-stent restenotic lesions in patients with coronary artery ...
Manufactured by:KLS Martin Group based inTuttlingen, GERMANY
The new marTract retractor system for minimally invasive coronary artery bypass (MIDCAB) includes two individually adjustable traction devices. For sufficient stability, a large amount of force is no longer required when pulling the rib arches and the system is easily ...
Manufactured by:Concept Medical Inc. based inTampa, FLORIDA (USA)
ABLUMINUS DES+ designed by propriety ENVISOLUTION technology in a way that address the unmet clinical needs specially in Diabetes Mellitus and Acute Myocardial infarction patients with Coronary Artery Disease. ABLUMINUS DES+ Receives on-label indication for Diabetes Mellitus and Acute Myocardial infarction. ...
Manufactured by:amg International GmbH based inWinsen, GERMANY
The ARTHOSPico is a multicellular Cobalt-Chromium stent that offers strength, stability, and flexibility for treating the most difficult lesions. The Co-Cr Stent is indicated for patients with coronary heart disease. It is inserted in coronary vessels with a diameter of 2.0 to 4.0 mm. It is used for the treatment of de-novo and restenotic lesions in ...
Manufactured by:Relisys Medical Devices Limited based inRanga Reddy, INDIA
Highlighting Features: • Good Curve Shape Memory • Good Torque control due to internal SS braid mesh • Very good kink Resistance • Excellent surface finish to prevent vascular spasm • Various tip configurations • Atraumatic soft tip to minimize the vessel damage • Straight and Angled curves designed for radial access of right and left coronary arteries. This ...