Refine by
Bloodstream Infection Equipment & Supplies In Malta
9 equipment items found
Manufactured by:Retractable Technologies, Inc. (RTI) based inLittle Elm, TEXAS (USA)
Patient Safe syringes are uniquely designed to reduce the risk of bloodstream infections resulting from catheter hub contamination. They protect both the patient and the clinician and are available in a variety of ...
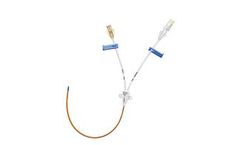
Manufactured by:Guangdong Baihe Medical Technology Co., Ltd. based inFoshan City, CHINA
The Catheter is made from premium silicone, which has excellent biocompatibility. The dacron cuff can protect immigration of catheter and reduce the chance of CRBI (Catheter Related Bloodstream Infections ). The silicone material makes catheter more floppy,hence it can minimize the trauma to the vein and reduce the incident of ...
Manufactured by:Attwill Medical Solutions based inLodi, WISCONSIN (USA)
The sterile, single-use disposable IV filter is infused with chlorhexidine gluconate (CHG) to help reduce the incidence of bloodstream infections, local infections and skin colonization in patients with IV catheters. This innovative product is designed as an in-line accessory for catheters to trap and kill microbial contaminants from catheter ...
Manufactured by:Vygon SAS based inEcouen, FRANCE
Antimicrobial catheter impregnated with Rifampicin (antibiotic) and Miconazole (antifungal), designed to reduce the incidence of catheter-related bloodstream infections. Active agents are incorporated in the structure of the catheter. Premistar has been registered for duration up to 30 ...
Manufactured by:Roche Diagnostics International AG based inRotkreuz, SWITZERLAND
GenMark’s ePlex® Blood-Culture Identification (BCID) Panels provide broad coverage of organisms that can lead to sepsis along with their resistance genes. This broad coverage means that about 95% of currently identified bloodstream infections can be detected early with the ePlex BCID Panels, compared to other panels that detect significantly fewer ...
Manufactured by:Accelerate Diagnostics, Inc. based inTucson, ARIZONA (USA)
Easier for the lab. Earlier for clinicians. Fast MIC results. Direct from positive blood cultures. Now with optional ID, and affordably ...
Manufactured by:Bio Fire Diagnostics based inSalt Lake City, UTAH (USA)
Early identification and treatment of sepsis are essential to combat one of the leading causes of hospital patient deaths.1 With 43 targets, the newly expanded and FDA-cleared BioFire® Blood Culture Identification 2 (BCID2) Panel detects pathogens and antimicrobial resistance genes directly from positive blood ...
Manufactured by:Cepheid based inSunnyvale, CALIFORNIA (USA)
On-demand molecular testing — an ideal solution: Accurate molecular detection of MRSA or SA from a gram-positive blood culture sample in about an hour; Simple test protocol can be run on all shifts to provide results around-the-clock; Provides clinicians actionable information when it is most needed; Easily integrates into your hospital’s bundle of sepsis ...
Manufactured by:Lepu Medical Technology (Beijing) Co.,Ltd. based inChangping District, CHINA
The Safecath Plus Antimicrobial Central Venous Catheter is developed by coating with anti-infectious drug on the basis of traditional CVC. It effectively reduces infection during placement and prolongs catheter indwelling time. It is mainly suitable for central venous administration, sampling and blood performance testing. The Catheter is made by exquisite processing of polyurethane and the ...