- Home
- Equipment
- netherlands
- uterine cavity
Refine by
Uterine Cavity Equipment & Supplies In Netherlands
12 equipment items found
Manufactured by:GTA S.r.l. based inQuistello, ITALY
Hysterosalpingography Catheter. HYSCA facilitates injection of contrast media into the uterine cavity ...
Manufactured by:Strauss Surgical LLC based inMiami, FLORIDA (USA)
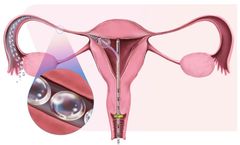
Achieve precise visualization and assessment of the uterine cavity with our 2.9mm diagnostic set. Designed for comfort and clarity, this slender instrument offers gentle access for minimally invasive procedures in outpatient and office ...
Manufactured by:JUNE Medical USA Inc based inNew Castle, DELAWARE (USA)
Wing Needle For Hysteroscopic Intrauterine Anaesthesia helps to reduce the pain associated with distension and / or manipulation of the uterine cavity during hysteroscopic gynaecology ...
Manufactured by:Femasys Inc. based inSuwanee, GEORGIA (US) (USA)
FemVue is the first FDA-cleared product that creates natural saline and air contrast and enables safe, reliable, and real time evaluation of the fallopian tubes with ultrasound. When performed with a uterine cavity assessment, a more comprehensive exam can be achieved from the comfort of the GYN’s ...
Manufactured by:JUNE Medical USA Inc based inNew Castle, DELAWARE (USA)
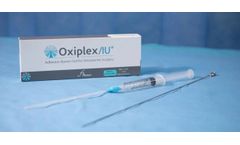
Flowable gel that is intended to serve as a temporary, absorbable mechanical barrier separating surgically traumatized opposing tissue surfaces in the uterine cavity where adhesions could potentially ...
Manufactured by:SMB Corporation Of India based inMumbai, INDIA
The Insertion Tube is equipped with a movable flange to aid in gauging the depth to which the Insertion Tube should be inserted through the cervical canal and into the uterine cavity. Centimeter Scale printed in blue is provided on the Insertion Tube for exact positioning of the flange on the Insertion Tube before Insertion. It is 99% effective Intrauterine ...
Manufactured by:Mona Lisa N.V. based inHerk-de-Stad, BELGIUM
This model is an improved rebuilt of the former, no more available “GyneT” with a new technology for quick and safe loading of the IUD. The classic T-shaped IUD is the most common used IUD-model in the world, recommended even by the WHO. With a copper surface of 380 mm² it shows the lowest pearl index among all copper ...
Manufactured by:LiNA Medical ApS. based inGlostrup, DENMARK
UNA Librata™ Endometrial Ablation Simplified; -2 minute thermal treatment time, Slim 5.4 mm catheter requires minimal or no dilation, Ideally suited for ambulatory gynecology, Only a simple sounding measurement ...
Manufactured by:SMB Corporation Of India based inMumbai, INDIA
Each SMB Cu 375 frame is made from compound of Low Density Polyethylene and Barium Sulphate (15% -24%) with two flexible arms with spurs. 99.99% purity OFE Copper wire (Diameter 0.40 mm ± 0.01 mm) is wound around the stem, giving a total copper surface area of 375mm2 ± 20mm2. The 'Frame' is equipped with Polyamide Monofilament(Suture)for checking the presence/position and easy ...
Manufactured by:SMB Corporation Of India based inMumbai, INDIA
Each SMB Cu 250 frame is made from compound of Low Density Polyethylene and Barium Sulphate(15% - 24%) with two flexible arms with spurs. 99-99% purity OFE Copper wire (Diameter 0.30 mm ± 0-01 mm) is wound around the stem, giving a total copper surface area of 250mm; ±20mm2. The 'Frame' is equipped with Polyamide Monofilament (Suture) for checking the presence/position of IUD and ...
Manufactured by:SMB Corporation Of India based inMumbai, INDIA
Each Y shaped SMB TCu 380Ag frame is made from compound of Low Density Polyethylene and Barium Sulphate (15% - 24%) wound with 0.40mm diameter copper wire with silver core of 0.1mm providing a surface area of 380mm2 ± 23mm2. The Y frame is equipped with Polyamide Monofilament thread (suture)for easy removal. It is packed together with an Insertion Tube and Rod in a Tyvek-Mylar film pouch ...
Manufactured by:Femasys Inc. based inSuwanee, GEORGIA (US) (USA)
FemBloc features our proprietary delivery platform, which places balloon technology close to the opening of both fallopian tubes. This in-office approach is designed to eliminate the risks of incisions, anesthesia, and hormones. As a nonsurgical procedure that is implant free, FemBloc delivers a biopolymer that is expected to expel within 3 months. Confirmation of procedure success can be ...