- Home
- Equipment
- north korea
- neurovascular
Show results for
Refine by
Neurovascular Equipment & Supplies In North Korea
44 equipment items found
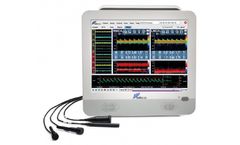
Manufactured by:SMT Medical Technology GmbH based inWuerzburg, GERMANY
Portable All-in-One TCD for Clinical and Scientific Applications. ...
Manufactured by:Terumo based inShibuya-ku, JAPAN
In collaboration with MicroVention*, a Terumo subsidiary with headquarters in the United States, the Neurovascular division provide interventional devices globally for the treatment of neurovascular conditions including brain aneurysm, stroke, and neurovascular malformations. We strive to develop innovative therapy devices that expand options for ...
Manufactured by:Mizuho America, Inc. based inUnion City, CALIFORNIA (USA)
The Neurovascular Bypass Instrumentation Set was designed for varying depths of cranial bypass. Designed in collaboration with Dr. Michael T. Lawton, MD. Useful in vascular, tumor, and spine ...
Manufactured by:NeuroSafe Medical Co., Ltd. based inZhuhai, CHINA
The DredgerTM Stent Retriever is a specialized medical device designed to rapidly address ischemic strokes by effectively removing thrombotic blockages in cerebral arteries. This device is crafted for use within eight hours of symptom onset, making it suitable for patients ineligible for, or unresponsive to, intravenous tissue plasminogen activator (IV t-PA) therapy. The stent retriever operates ...
Manufactured by:Mizuho Medical Co., Ltd based inTokyo, JAPAN
Over 40 instruments designed for varying depths of cranial bypass ...
Manufactured by:Ilion Medical Inc. based inMineapolis, MINNESOTA (USA)
Ilion’s surgical system features the NADIA NeuroSafe posterior bridging approach which eliminates neurovascular risks commonly associated with Laterally-Based Techniques. Our posterior approach never crosses critical neurovascular ...
Manufactured by:NeuroSafe Medical Co., Ltd. based inZhuhai, CHINA
The Lava Liquid Embolic System is formulated for effective embolization of brain arteriovenous malformations and hypervascular tumors. This injectable radiopaque embolic agent comprises EVOH (ethylene vinyl-alcohol copolymer), DMSO (dimethyl-sulfoxide), and micronized tantalum powder, facilitating exceptional visibility under fluoroscopy for precise delivery. The system provides a range of ...
Manufactured by:Genesis MedTech based inSingapore City, SINGAPORE
No damage to blood vessels, operation is more ...
Manufactured by:Surmodics, Inc. based inEden Prairie, MINNESOTA (USA)
The Surmodics low-particulate lubricious coatings have a proven track record, meeting the demanding regulatory requirements in all clinical segments: coronary, peripheral, neurovascular, and structural heart devices. Our covalently bound family of Hydrophilic Coating formulations demonstrate markedly superior lubricity and tracking, durability, and particulate ...
Manufactured by:LVD Biotech based inSant Vicenç dels Horts, SPAIN
The most common applications in the medical field are the shaft of catheters, guidewire lumen and transitions, for the coronary, peripheral and neurovascular fields. ...
Manufactured by:LVD Biotech based inSant Vicenç dels Horts, SPAIN
The most common applications in the medical field are the shaft of catheters, a guidewire lumen and transitions, for the coronary, peripheral and neurovascular ...
Manufactured by:SMT Medical Technology GmbH based inWuerzburg, GERMANY
Digital extra- and transcranial Doppler; Neurovascular Testing in your daily clinical and office ...
Manufactured by:LVD Biotech based inSant Vicenç dels Horts, SPAIN
The most common applications in the medical field are reinforced catheters, guiding catheters, micro-catheters, thrombus extraction catheters, and support catheters, for coronary, peripheral, and neurovascular ...
Manufactured by:LVD Biotech based inSant Vicenç dels Horts, SPAIN
The most common applications in the medical field are shaft catheters with inflation channel integrated, steerable catheters, and electronic sensors channels for the coronary, peripheral and neurovascular ...
Manufactured by:Confluent Medical Technologies based inScottsdale, ARIZONA (USA)
Inclusions can be point of fracture initiation if located at a high strain area of the component. Ideal for high fatigue applications such as Cardiovascular Implants, or small feature components such as Neurovascular Stents. CT Scan images show Nitinol Tubing Cross Section comparison of SE508 (ASTM Standard Inclusion Rate) vs SE508-ELI (High Purity ...
Manufactured by:Biomerics based inSalt Lake City, UTAH (USA)
We specialize in the design, development, and manufacturing of complex, highly specialized catheters, steerable devices, and delivery systems for interventional markets. We’ve worked with customers on steerable balloon catheters, steerable neurovascular devices, and delivery devices for structural heart implants, to name a few. As your vertically integrated partner, we have ...
Manufactured by:MB Medical Braider based inDongguan City, CHINA
Our Catheter Braiding Machine is designed for the production of braid-reinforced catheter shaft which has applications in: Interventional Vascular, Neurovascular, Electrophysiology, Endo-surgery, Diagnostic, and other minimally invasive applications. With the Schneider control system, our braiding machine provides our customers best in class braiding solution which improves the ...
Manufactured by:Emulate based inBoston, MASSACHUSETTS (USA)
Research mechanisms of neuroinflammation and evaluate the effect of therapeutics in a comprehensive model of the neurovascular ...
Manufactured by:Tekni-Plex based inWayne, PENNSYLVANIA (USA)
Our specialty portfolio supports minimally-invasive delivery systems for the cardiovascular, electrophysiology, neurovascular, gastrointestinal/endoscopy, structural heart and interventional radiology medical markets. Products include single and multi-lumen, coextruded, striped or braided tubing. We also include market-leading acetal mandrels used for manufacturing ...
Manufactured by:Bioplate based inPlacentia, CALIFORNIA (USA)
Warnings include avoiding repeated sterilization and maintaining sterility of packages. Potential risks include infection, scaffold material reactions, and neurovascular issues, emphasizing the importance of following surgical ...