Show results for
Refine by
Intravenous Anesthesia Tiva Administration Equipment & Supplies In Peru
73 equipment items found
Manufactured by:Healthline Medical Products based inSalt Lake City, UTAH (USA)
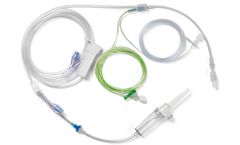
Gravity IV Administration Set, with Green Striped & Clear Minibore Line. The use of administration sets specifically designed for TIVA is recommended. Easy to use and promotes accurate delivery of drugs introduced closer to the patient’s IV access. ...
Manufactured by:eTheRNA based inNiel, BELGIUM
Therapeutic area: liver diseases, secreted factors; High expression in liver after intravenous ...
Manufactured by:TSE Systems GmbH based inBad Homburg, GERMANY
We offer complete setups for Intravenous self-administration in both rats and mice. Choose from INSTECH precision stainless steel or disposable plastic swivels available in a range of tubing sizes and channel numbers (with optional electrical channels). Other products are head block tethers, button and jacket tethers, infusion harnesses, a variety of swivel mounts and strain relief adapters as ...
Manufactured by:B. Braun Medical Inc. based inBethlehem, PENNSYLVANIA (USA)
Dextrose and Sodium Chloride Injections USP are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents. These products are intended for intravenous administration. These intravenous solutions are indicated for use in adults and pediatric patients as sources of electrolytes, calories and water for hydration. Not made with latex, PVC or ...
Manufactured by:B. Braun Medical Inc. based inBethlehem, PENNSYLVANIA (USA)
Dextrose and Sodium Chloride Injections USP are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents. These products are intended for intravenous administration. These intravenous solutions are indicated for use in adults and pediatric patients as sources of electrolytes, calories and water for hydration. Not made with latex, PVC or ...
Manufactured by:B. Braun Medical Inc. based inBethlehem, PENNSYLVANIA (USA)
Dextrose and Sodium Chloride Injections USP are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents. These products are intended for intravenous administration. These intravenous solutions are indicated for use in adults and pediatric patients as sources of electrolytes, calories and water for hydration. Not made with latex, PVC or DEHP. Specific Gravity: ...
Manufactured by:B. Braun Medical Inc. based inBethlehem, PENNSYLVANIA (USA)
Dextrose and Sodium Chloride Injections USP are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents. These products are intended for intravenous administration. These intravenous solutions are indicated for use in adults and pediatric patients as sources of electrolytes, calories and water for hydration. Not made with latex, PVC or ...
Manufactured by:B. Braun Medical Inc. based inBethlehem, PENNSYLVANIA (USA)
Dextrose and Sodium Chloride Injections USP are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents. These products are intended for intravenous administration. These intravenous solutions are indicated for use in adults and pediatric patients as sources of electrolytes, calories and water for hydration. Not made with latex, PVC or ...
Manufactured by:Biotest AG based inDreieich, GERMANY
At the ISTH 2020 Biotest presented its novel recombinant Factor VIII molecules overcoming the major challenges of current Factor VIII replacement therapy by combining a significantly extended half-life, low immunogenicity and the possibility of either subcutaneous or intravenous ...
Manufactured by:Piramal Critical Care (PCC) based inBethlehem, PENNSYLVANIA (USA)
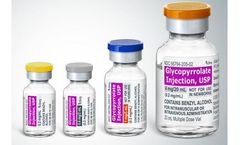
Glycopyrrolate Injection, USP is a synthetic anticholinergic agent. Each 1 mL contains: Glycopyrrolate, USP 0.2 mg Water for Injection, USP q.s. Benzyl Alcohol, NF 0.9% (preservative) pH adjusted, when necessary, with hydrochloric acid. For Intramuscular (IM) or Intravenous (IV) administration. Glycopyrrolate is a quaternary ammonium salt with the following chemical name: ...
Manufactured by:Nephron Pharmaceuticals Corporation based inWest Columbia, SOUTH CAROLINA (USA)
Item No. : 69374-913-10. Dosage Form : Injection. Strength : 100 mg/mL. Content Volume : 10 mL. Primary Packaging : Syringe. Administration : Intravenous. Delivery System : Single Dose. Retail Packaging : Carton. Syringe per Carton : 5. Carton per Case : 6. ...
Manufactured by:Octapharma AG based inLachen, SWITZERLAND
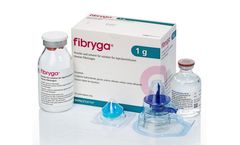
Fibryga® is a highly purified and pathogen inactivated human fibrinogen concentrate and an effective and convenient treatment for fibrinogen deficiency. Fibryga offers a number of benefits: concentration is standardised; it is stable at room temperature, can be reconstituted within minutes, and has a low infusion volume; there is no need for thawing or cross-matching blood type and it is ...
Manufactured by:B. Braun Medical Inc. based inBethlehem, PENNSYLVANIA (USA)
Isolyte P in 5% Dextrose is sterile, nonpyrogenic, and contains no bacteriostatic or antimicrobial agents or added buffers. This product is intended for intravenous administration. This solution is indicated for use in adults as a source of electrolytes, calories and water for hydration, and as an alkalinizing agent. Not made with latex, PVC or DEHP. ...
Manufactured by:Biotest AG based inDreieich, GERMANY
Trimodulin (BT588, predecessor BT086) is a human plasma-derived native polyvalent antibody preparation for intravenous administration. Trimodulin contains immunoglobulins IgM (-23%), IgA (~ 21%) and igG (-56%). Trimodulin mediates its mode of action via three potential mechanisms which are; opsonization of causal pathogens, neutralizing of microbial pathogens and their virulence factors (endo- ...
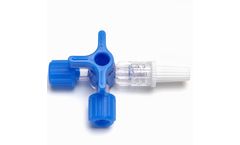
Manufactured by:CV Médica, SL based inSarral (Tarragona), SPAIN
3-way stop cock with two female and one male luer-lock connections to control two infusion/extraction ports via a single lancing device or lane. Sterilized with ethylene oxide gas, pursuant to EN ...
Manufactured by:eTheRNA based inNiel, BELGIUM
Therapeutic area: cancer vaccines; Systemic, intravenous (IV) ...
Manufactured by:SEQENS Group based inÉcully, FRANCE
Cisatracurium is a nondepolarizing skeletal muscle relaxant for intravenous administration. Cisatracurium acts on cholinergic receptors, blocking neuromuscular transmission. Cisatracurium is one of the ten isomers of the parent molecule, atracurium. Cisatracurium represents approximately 15% of the atracurium ...
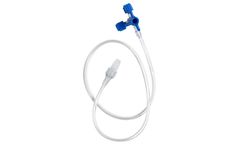
Manufactured by:CV Médica, SL based inSarral (Tarragona), SPAIN
Extension line with 3-way stopcock with two female and one male luer-lock connections to control two infusion/extraction ports through a unique puncture device. Sterilized with ethylene oxide gas, according to EN ...
Manufactured by:B. Braun Medical Inc. based inBethlehem, PENNSYLVANIA (USA)
Available in 250 mL, 500 mL and 1000 mL EXCEL IV containers and 100 mL and 150 mL PAB Partial Additive containers. Dextrose Injections USP are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents. These products are intended for intravenous administration. These intravenous solutions are indicated for use in adults and pediatric patients as sources of calories and water for ...
Manufactured by:InSilicoTrials Technologies based inStafford, VIRGINIA (USA)
A tool that performs in silico clinical trials to assess the neutropenic effects of a chemotherapeutic agent in a virtual population of cancer patients ...