Show results for
Refine by
Cancer Drive Gene Mutations Detection Equipment & Supplies In Romania
54 equipment items found
Manufactured by:Coburger Lehrmittelanstalt based in, GERMANY
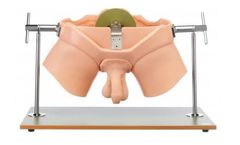
Produced in co-operation with Prof. Dr. J. Sökeland, Dortmund. Made of special plastic. An urological practice model that allows rectal examinations and palpation of the testicles to be learned under true-to-life ...
Manufactured by:ScreenPoint Medical based inNijmegen, NETHERLANDS
The resulting product, Transpara, has featured in multiple peer-reviewed publications that show it enables significant clinical improvements to the timely diagnosis of breast cancer. “Transpara is now being used by radiologists in clinical practice, but this is only the beginning! We are constantly innovating and we will continue to help doctors to achieve higher breast cancer survival ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Empower is a genetic test for those who want to know more about their risk of developing cancer, why it might be common in their family, or want to inform treatment options following a cancer diagnosis. Empower screens for genes associated with increased risk for common hereditary cancers. Our Empower multi-cancer panels include commonly screened-for genes associated with 12+ types of ...
Manufactured by:OTraces Inc. based inSykesville, MARYLAND (USA)
BC Sera Dx Immunoassay test panel for detection of the presence of breast cancer in women. In the development pipeline are an Ovarian, OV Sera Dx, and Lung, LG Sera Dx, cancer detection test ...
by:Acuamark Diagnostics based inNew York, NEW YORK (USA)
The vast majority of cancer cases are detected at stage III or beyond, resulting in poor cancer survival of ~50% in the aggregate. Cancer cases are forecasted to reach 25M per year over the next two decades, causing deaths to rise to 13M per year (worldwide). Annually, the worldwide economic impact of cancer is estimated by the WHO at $1.2 trillion. ...
Manufactured by:PREVENTIS GMBH based inBensheim, GERMANY
The immunological occult blood test PreventID® CC is a rapid test for self-testing for the qualitative detection of the valuable red blood pigment hemoglobin in stool as a marker for intestinal bleeding. The result can be read after 10 minutes. According to the WHO, colorectal cancer is the third most common cancer worldwide, with approximately 1.9 million new cases in 2020. In Germany ...
Manufactured by:PREVENTIS GMBH based inBensheim, GERMANY
The immunological occult blood test PreventID® CC is a rapid test for self-testing for the qualitative detection of the valuable red blood pigment hemoglobin in stool as a marker for intestinal bleeding. The result can be read after 10 minutes. ...
Manufactured by:IMB Dx, Inc. based inSeoul, SOUTH KOREA
AlphaLiquid® Screening is a blood-based test for early detection of multiple cancers. With 10mL of blood, the test analyzes genetic and epigenetic features in cfDNA to screen for signals of cancer as well as predict the tissue of origin. ...
Manufactured by:VIDAR Systems Corporation based inHerndon, VIRGINIA (USA)
The CAD PRO® Advantage film digitizer is specifically designed for mammography CAD. CAD systems incorporate advanced pattern recognition and image analysis, requiring a film digitizer that meets the highest standards of image quality and reliability. These advanced systems enable radiologists to improve their ability to detect early stage cancers in an efficient manner. The CAD PRO Advantage ...
Manufactured by:Universal Diagnostics SL based inSeville, SPAIN
We are developing Signal-X, a multi-cancer platform able to detect various types of high-burden cancers with high sensitivity and tissue-of-origin specificity. ...
Manufactured by:Virasoft Inc. based inSarıyer, TURKEY
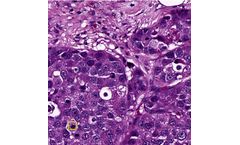
In reporting of breast cancer pathology, mitosis detection and counting of mitotic cells are important factors that affect the tumor grade and the treatment of the patient. Mitotic cell counting requires standardization to a fixed field area. The total number of mitoses per 10 HPF is recorded. Normally, the count is performed manually by pathologists, but automating the process could reduce its ...
by:Ezra based inGeorgetown, TEXAS (USA)
Ezra offers advanced MRI-based full-body scans aimed at early cancer detection. The service employs 3 Tesla (3T) MRI technology, known for its higher-resolution imaging and superior signal-to-noise ratio, alongside 1.5 Tesla (1.5T) MRI for broader accessibility. These scans are designed to identify potential early-stage cancers, significantly improving survival rates. Due to telehealth ...
Manufactured by:3B Pharmaceuticals GmbH based inBerlin, GERMANY
Radiopharmaceuticals are unique medicinal formulations containing radioisotopes which are used in major clinical areas for diagnosis and/or therapy. Targeted radiopharmaceuticals bind to specific structures such as proteins or cells within the body and thus accumulate in particular places, such as e.g. tumor lesions in a cancer ...
by:GE HealthCare based inChicago, ILLINOIS (USA)
The Invenia ABUS 2.0 is designed as an FDA-approved ultrasound supplemental screening technology, aiming to improve cancer detection in women with dense breast tissue. It offers a 35.7% increase in detection over traditional mammography, thanks to its superior image quality and advanced interpretation tools. The system integrates AI assistance to enhance clinical confidence by detecting and ...
Manufactured by:Twist Bioscience based inSouth San Francisco, CALIFORNIA (USA)
NGS-based liquid biopsy is a rapidly evolving application that requires accurate and precise reference standards. The Twist Pan-cancer Reference Standard is a high-quality, standardized control for use in the research and development of NGS-based liquid biopsy assays. This reference standard is ideal for establishing the analytical limit of detection (LoD) for specific cancer variants and as a ...
Manufactured by:Nucleix Ltd. based inSan Diego, CALIFORNIA (USA)
Bladder cancer is the 5th most common cancer in the western world, and 70%-80% of the patients are diagnosed with non-muscle invasive disease. There are estimated 400,000 to 800,000 active bladder cancer patients in the United States, as well as 1 to 2 million in Europe, who undergo local resection of the tumor and then have 1-4 follow-up visits each year due to the high recurrence rate of the ...
Manufactured by:Epigenomics AG based inBerlin, GERMANY
Colorectal cancer is curable in about 90% of cases if detected early. Nevertheless, this disease is one of the most frequent causes of cancer-related deaths in the industrialized world. Regular screening can prevent colorectal cancer. Epi proColon® offers a convenient way of detecting colorectal cancer: in blood. It’s as simple as a blood draw. The test is performed by a clinical ...
Manufactured by:Hybribio based inKowloon Bay, HONG KONG
The Stool DNA Methylation Real-time PCR Kit serves as a diagnostic tool for individuals at high risk of colorectal cancer, facilitating in vitro qualitative detection of methylated SDC2 and TFPI2 genes within intestinal exfoliated cells. Designed for efficacy and user-friendliness, this kit enables easy operation while maintaining a short processing time without the unpleasant odor associated ...
Manufactured by:Nucleix Ltd. based inSan Diego, CALIFORNIA (USA)
Lung cancer remains a leading cause of death from cancer globally, due to its high incidence and late-stage diagnosis. Most patients are currently diagnosed with late-stage cancer, though research has shown 5-year survival rates are up to 10 times greater when disease is detected early. Annual imaging (low-dose CT scans) is recommended for screening individuals with highest risk, but less than ...
Manufactured by:Rarecells Diagnostics SAS based inParis Cedex 06, FRANCE
Cancer is the second cause of death, accounting for nearly 9 million deaths in 2017 all over the world. In 2019, 1,762,450 new cancer cases and 606,880 cancer deaths are projected to occur in the United States. It is estimated that metastasis is the primary cause of 90% of all cancer-related deaths. Circulating Tumor Cells (CTCs) detection and analysis are the foundation of non-invasive tests ...