Refine by
Nasal Swab Collection Equipment & Supplies In Russia
16 equipment items found
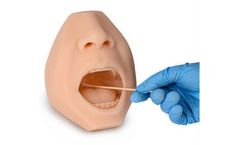
Manufactured by:Pro-Health Product Ltd based inGuangzhou, CHINA
The must-have trainer is ideal for use in visual demonstration and training of a nasal & oropharyngeal swab sample collection not only for the test and diagnosis of COVID-19 but also for many respiratory viruses and airborne ...
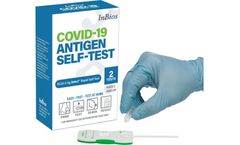
Manufactured by:InBios International, Inc based inSeattle, WASHINGTON (USA)
The SCoV-2 Ag Detect™ Rapid Self-Test’s patent pending design has been developed to be incredibly simple to use. It uses a shallow nasal swab (inserted just one-half inch) and offers easy-to-read results – all in the time it takes to drink your morning latte, go for a mile run or read a couple of chapters in your latest novel. ...
Manufactured by:Mediblink d.o.o. based inTrebnje, SLOVENIA
The aspirator offers both a handy design and is safe and easy to use. Two different sizes of soft silicone nasal tips are enclosed, which enable safe product use. It offers a twelve chord melody music option, to create a pleasant user experience for your baby/child when using the aspirator. The nasal tips and a collection cup are removable and can simply be cleaned under running water. Even ...
Manufactured by:Ellume Limited based inEast Brisbane, AUSTRALIA
Our patented detection method uses unique fluorescent nanoparticles and a sophisticated reader system to achieve accurate results from a clinical sample within minutes. We create efficiencies using our platform technology across a range of diagnostic tests for consumers, healthcare professionals at the point-of-care and higher-throughput laboratories. Our strategy of using digital technology at ...
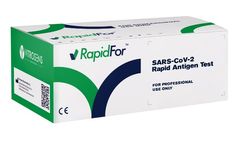
Manufactured by:Vitrosens Biotechnology A.S. based inÜmraniye, TURKEY
RapidFor™ SARS-CoV-2 Rapid Antigen Test Kit enables in vitro qualitative detection of SARS-CoV-2 antigen, which results in COVID-19 infection. The RapidFor™ SARS-CoV-2 Rapid Antigen Test Kit uses the colloidal label as a tool for SARS-CoV-2 antigen detection in the collected nasopharyngeal, nasal, and oropharyngeal ...
Manufactured by:Genabio Diagnostics Inc. based inBedford, MASSACHUSETTS (USA)
Intended use: iHealth® COVID-19 Antigen Rapid Test is a lateral flow assay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2. This test is authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 15 years or older with symptoms of COVID-19 ...
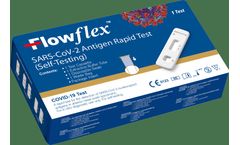
Manufactured by:AusDiagnostics based inMascot, AUSTRALIA
The Flowflex™ SARS-CoV-2 Antigen Rapid Test (Self-testing) allows qualitative detection of the SARS-CoV-2 nucleocapsid antigen in just 15 minutes. Designed to be easy-to-use and rigorously tested, the Flowflex™ self-test kit is trusted by major clinics around the world to provide accurate detection of SARS-CoV-2. ...
Manufactured by:Chembio Diagnostic Systems, Inc. based inHauppauge, NEW YORK (USA)
Status Flu A & B is a CLIA Waived in vitro rapid qualitative test that detects Influenza Types A and B antigens directly from nasal or nasopharyngeal swab ...
Manufactured by:Ellume Limited based inEast Brisbane, AUSTRALIA
Ellume’s swab+drop COVID-19 Self-Swab Kit allows for the self-collection of nasal samples based on easy to follow, animated step-by-step online instructions. The swab+drop Self-Swab Kit has an entry on the Australian Register of Therapeutic Goods (ARTG). ...
Manufactured by:Biomed Diagnostics, Inc. based inWhite City, OREGON (USA)
Pediatric, Mid-Turbinate Flocked Nasal Swabs with Allium™ Flock Technology. Individually-sealed, sterile, flocked swabs with bear face shaped stopper and breakable shaft. For collecting SARS- CoV-2 and other clinical specimens with patient and physician comfort in ...
by:Thermo Fisher Scientific, LIMS & Laboratory Software based inPhiladelphia, PENNSYLVANIA (USA)
SpeciMAX Saliva Collection Kit is optimized to easily collect and transport saliva samples for SARS-CoV-2. Improve your high throughput SARS-CoV-2 surveillance testing with the SpeciMax Saliva Collection Kit. Your lab’s solution for efficient, cost-effective, standardized saliva collection compatible with downstream analysis workflows and automation ...
Manufactured by:RapiGEN Inc. based inSuwon-si, SOUTH KOREA
One Step SARS-CoV-2 Antigen Rapid Test. BIOCREDIT COVID-19 Ag Home Test Nasal is a rapid chromatographic immunoassay intended for the qualitative detection of SARS-CoV-2 in individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection. This test is authorized for home use with self-collected direct nasal ...
Manufactured by:RapiGEN Inc. based inSuwon-si, SOUTH KOREA
One Step SARS-CoV-2 Antigen Rapid Test. BIOCREDIT COVID-19 Ag Home Test Nasal is a rapid chromatographic immunoassay intended for the qualitative detection of nucleocapsid (N) protein antigen from SARS-CoV-2 in individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection. This test is authorized for home use with ...
Manufactured by:Gold Standard Diagnostics based inDavis, CALIFORNIA (USA)
The GSD NovaGen SARS-CoV-2 Ag Rapid Test (Nasal Swab) is a single-use test kit intended to detect the presence of the SARS-CoV-2 virus, with self-collected nasal swab specimen from symptomatic individuals who are suspected of being infected with COVID-19. A positive result indicates SARS-CoV-2 antigens present in ...
Manufactured by:Gold Standard Diagnostics based inDavis, CALIFORNIA (USA)
The Antigen Rapid Test assay from the Gold Standard Diagnostics Group is a rapid chromatographic immunoassay for the qualitative detection of SARS-CoV-2 Nucleocapsid (N) Protein antigens present in respiratory ...
Manufactured by:Access Bio based inSomerset, NEW JERSEY (USA)
The CareStart™ COVID-19 Antigen Test is a lateral flow immunochromatographic assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal or anterior nasal swab specimens directly collected from individuals who are either suspected of COVID-19 by their healthcare provider within the ...