Show results for
Refine by
Applications
- Microbiome Therapeutics for Patients and Physicians
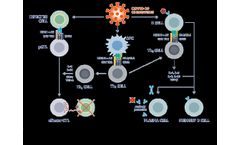
- Vaccine for COVID-19
- BSD-2000 - Deep Regional Hyperthermia System for Clinical
- Medical cannabis solutions for regulatory pathway sector
- Medical cannabis solutions for mental health costs sector
- IONICON PTR-TOF instruments deployed for COVID-19 detection in breath
- Drug Dolution for Liver Metastases
- Drug Dolution for Gastric Cancer
- Microbiota for Restoration Therapy
- Microbiota Based Product for Physician-Sponsored Studies
- Solution for Clinical Trials
- Solution for Access to Investigational Medicines
- Liquid Biopsy Technology for Hereditary Cancer Screening
Clinical Phases Equipment & Supplies In Serbia
156 equipment items found
Manufactured by:Gesynta Pharma AB based inStockholm, SWEDEN
Gesynta Pharma’s drug candidate GS-073 is expected to enter clinical phase I in 2022. It is currently in the final stages of preclinical development. This project will be addressing an indication with high unmet need, which is separate from systemic ...
Manufactured by:BCN Peptides S.A. based inSant Quintí de Mediona, SPAIN
BCN Peptides ensures the highest qualities and shortest times thanks to our experience in the manufacturing of peptides for all clinical phases, from pre-clinical to phase I, II, III and commercial stage. Our facility has been specially designed by BCN Peptides applying our expert know-how in Solid Phase Peptide ...
by:Verb Biotics based inBoston, MASSACHUSETTS (USA)
Using a revolutionary form of living biotherapy targeting the lung, YSOPIA is pioneering the innovative field of the lung microbiome. As part of this program, YSOPIA has selected a clinical candidate having demonstrated strong in vivo efficacy on inflammation and lung functions. We have achieved the formulation feasibility and cleared the regulatory hurdles associated with ...
Manufactured by:iMouse GmbH based inBerlin, GERMANY
Its intelligent Vision AI and machine learning platform aids scientists in refining the prediction of new drug candidates, enhancing translation from preclinical to clinical phases while minimizing failure ...
Manufactured by:Gesynta Pharma AB based inStockholm, SWEDEN
GS-248 is Gesynta Pharma’s most advanced drug candidate, currently in clinical development as a potential treatment of systemic sclerosis. Recent clinical phase I data in healthy individuals show that GS-248 potently inhibits the production of PGE2 in a tolerable and safe manner, while increasing the observed levels of protective and ...
Manufactured by:Virometix AG based inSchlieren, SWITZERLAND
With V-306 Virometix offers a differentiated approach to RSV vaccine development. Our vaccine candidate – already in clinical phase I – demonstrates strong, durable, specific and protective immune responses, with minimal risk of vaccine-associated enhanced respiratory disease ...
Manufactured by:CanaQuest Medical Corp. based inMississauga, ONTARIO (CANADA)
Clinical testing on humans can only begin after a pre-clinical phase, involving laboratory studies (in vitro) and tests on animals, which has shown that the experimental drug is considered safe and effective. However, no animal is sufficiently similar to humans (even genetically modified ones) to make human testing unnecessary. ...
Manufactured by:OSE Immunotherapeutics based inNantes, FRANCE
CoVepiT, a multi-target and multi-variant vaccine candidate against COVID-19. CoVepiT is a next-generation multi-target, multi-variant vaccine against SARS-CoV-2 in clinical Phase 1. The vaccine candidate was designed using optimized epitopes selected after screening more than 67,000 global SARS-CoV-2 genomes, as well as those of previous human-infective CoVs, ...
Manufactured by:Addex Therapeutics based inGeneva, SWITZERLAND
Our partner, Indivior, has licensed worldwide rights to our GABAB PAM program and is responsible for all development, manufacture and commercialization of any selected GABAB PAM drug candidates. Under the agreement, we are responsible for executing a research program funded by Indivior to discover novel drug candidates. Indivior has the right to select GABAB PAM drug candidates from our research ...
by:STALICLA based inGeneve, SWITZERLAND
STALICLA is advancing a robust pipeline of both proprietary and partnered therapeutic ...
Manufactured by:Shanghai Henlius Biotech, Inc based inShanghai, CHINA
It is potentially indicated for the treatment of postmenopausal women with OP at high risk for fracture. First subject has been doesd in the international multi-centre phase 3 clinical trial in China for the treatment of postmenopausal osteoporosis in women with high fracture ...
Manufactured by:Poietis based inPessac, FRANCE
NGB-C is a clinical-grade, GMP-compliant Bioprinter designed to meet the needs of Advanced Therapy Medicinal Products (ATMP) production and the requirements of Poietis’ ...
by:Vivozon Inc. based inYongin-si, SOUTH KOREA
A multicenter, randomized, double-blind, parallel-group, placebo-controlled phase 3 clinical trial to evaluate the analgesic efficacy and safety of VVZ-149 injection in patients who have pain after laparoscopic ...
by:Genmab A/S based inCopenhagen V, DENMARK
DuoBody-CD40x4-1BB (GEN1042) is a proprietary bispecific antibody, jointly owned by Genmab and BioNTech, created using Genmab’s DuoBody® technology. It is being co-developed under an agreement in which the companies share all costs and future profits for the product on a 50:50 basis. CD40 and 4-1BB were selected as targets to enhance both dendritic cells (DC) and antigen-dependent ...
Manufactured by:Addex Therapeutics based inGeneva, SWITZERLAND
We are developing muscarinic acetylcholine receptor subtype 4 positive allosteric modulator, or M4 PAM, as a novel orally available treatment for schizophrenia & other psychosis. Schizophrenia affects approximately 24 million people worldwide, requiring lifelong treatment to reduce severe impact on their everyday life. The market for antipsychotics represents over $7B annual sales in the ...
by:Horizon Therapeutics plc based inSaint Kevin`s, IRELAND
We are investigating daxdilimab in dermatomyositis, discoid lupus erythematosus and lupus nephritis as well conducting Phase 2 clinical trials in alopecia areata and systemic lupus ...
Manufactured by:Shanghai Henlius Biotech, Inc based inShanghai, CHINA
HLX04-O, recombinant anti-VEGF humanised monoclonal antibody injection. It is indicated for wet age-related macular degeneration (wAMD). HLX04-O was granted Phase 3 clinical study approvals in Australia, the United States, Singapore and the EU countries such as Latvia, Hungary and Spanish. In April 2022, the first patient in Australia and Latvia was dosed in the ...
Manufactured by:Shanghai Henlius Biotech, Inc based inShanghai, CHINA
HLX07, recombinant anti-epidermal growth factor receptor (EGFR) humanised monoclonal antibody injection independently researched and developed as biobetter. It was granted IND approvals to be evaluated in clinical trials in China and the United States. It demonstrated favorable safety and tolerability profile in a prospective, open-label, dose-escalation Phase 1 ...
Manufactured by:ViruSure GmbH based inVienna, AUSTRIA
Recombinant proteins are proteins expressed in cell lines which have been genetically modified by DNA recombinant technology. These therapeutics include recombinant blood clotting factors, hormones, enzymes, interferons, growth factors and other bioengineered ...
Manufactured by:Addex Therapeutics based inGeneva, SWITZERLAND
These indications have been validated with baclofen, an orthosteric agonist of GABAB and present a significant unmet medical need and commercial opportunity. We are in clinical candidate selection phase and expect IND enabling studies to be initiated in ...