- Home
- Equipment
- turkmenistan
- cell free dna
Refine by
Cell Free Dna Equipment & Supplies In Turkmenistan
24 equipment items found
Manufactured by:Streck, Inc. based inLa Vista, NEBRASKA (USA)
Cell-Free DNA BCT is a direct-draw venous whole blood collection device intended for the collection, stabilization and transport of venous whole blood samples for use in conjunction with cell-free DNA next generation sequencing liquid biopsy assays that have been cleared or approved for use with ...
Manufactured by:Epigenomics AG based inBerlin, GERMANY
High-quality cell-free DNA is easily and robustly purified from up to 3,5 ml of plasma. Streamlined procedure for fast and reliable results, Complete cytosine conversion, DNA extraction system for highly sensitive ...
by:CareDx, Inc based inSouth San Francisco, CALIFORNIA (USA)
AlloSeq cfDNA is a minimally invasive blood test that measures the amount of donor-derived cell-free DNA (dd-cfDNA) for transplant surveillance. It is currently only available outside of the United ...
Manufactured by:SiO2 Materials Science based inAuburn, ALASKA (USA)
The company creates engineered blood collection tubes for the Genomics & Diagnostics market. Specifically, the SiO2 blood collection tubes capture and preserve cell free DNA, cell free RNA, and cancer tumor cells using a single blood sample. Our isolation and preservation technology are a ...
Manufactured by:Oasis Diagnostics based inVancouver, WASHINGTON (USA)
The Pure•SAL Oral Specimen Collection Kit provides a simple non-invasive and rapid platform for isolating “liquid biopsy” specimens including cell free DNA, cell free RNA, exosomes, or proteins in a single step. The harvested, purified saliva specimen is stabilized if required and available for ...
Manufactured by:IMB Dx, Inc. based inSeoul, SOUTH KOREA
Cell-free DNA (cfDNA) is circulating fragments of DNA that is released from cells into the circulatory system throughout the body. Tumor cells also release DNA into the blood stream, which is called circulation-tumor DNA(ctDNA). IMBdx ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Covered by Medicare, Prospera is a transplant rejection assessment test that uses a simple blood draw to evaluate the risk of rejection of a transplanted kidney. Through the use of advanced cell-free DNA technology, Prospera increases a provider’s ability to identify otherwise undetected rejection that might lead to kidney loss. ...
Manufactured by:Xpedite Diagnostics based inHallbergmoos, GERMANY
The SwiftX™ cfDNA extraction kit revolutionizes the process of isolating cell-free DNA from various bodily fluids such as serum, plasma, and urine in under 15 minutes. Designed to cater to the needs of researchers in oncology, prenatal testing, and infectious disease studies, SwiftX™ offers an efficient alternative to traditional ...
Manufactured by:Xpedite Diagnostics based inHallbergmoos, GERMANY
The SwiftX™ cfDNA Extraction Kit is designed for efficient extraction of cell-free DNA (cfDNA) from large volumes of serum, plasma, and urine samples in under 15 minutes. This kit is particularly beneficial for applications in oncology research, prenatal testing, and infectious disease studies due to its rapid, sensitive, and accurate cfDNA ...
Manufactured by:Oasis Diagnostics based inVancouver, WASHINGTON (USA)
RNAPro•SAL was developed as an easy to use and cost effective tool for the split sample collection of rich sources of RNA miRNA, mRNA and proteins found in saliva. The proprietary RNAPro•SAL kit provides two equivalent samples of saliva which total 1.0 mL of saliva in 1-3 minutes. These may be analyzed later for either RNA or protein components. A unique built in Sample Volume Adequacy ...
Manufactured by:Twist Bioscience based inSouth San Francisco, CALIFORNIA (USA)
NGS-based liquid biopsy is a rapidly evolving application that requires accurate and precise reference standards. The Twist Pan-cancer Reference Standard is a high-quality, standardized control for use in the research and development of NGS-based liquid biopsy assays. This reference standard is ideal for establishing the analytical limit of detection (LoD) for specific cancer variants and as a ...
Manufactured by:Biolidics Limited based inBizCentre, SINGAPORE
Our ClearCell FX1 System is a fully automated device for cell retrieval that can separate and enrich wholly intact and viable circulating tumour cells, or CTCs from small amounts of blood. It is driven by our CTChip®FR1 biochip, which is a single-use microfluidic ...
Manufactured by:Stilla Technologies based inVillejuif, FRANCE
Get ready-to-use, fully-validated panels for the detection and quantification of nucleic acids. Crystal Digital PCR™ kits allows researchers to detect and quantify nucleic acids with ready-to-use, pre-designed assays for fully-validated target panels. These digital PCR panel kits are specially developed by Stilla Technologies and its partners ID Solutions. For fully-customizable kits and/or ...
Manufactured by:BAG Diagnostics GmbH based inLich, GERMANY
Rhesus prophylaxis rethought! FastQ® RHD fetal (RUO) - for targeted rhesus prophylaxis. Thanks to our new real-time PCR kit, targeted anti-D prophylaxis is possible through the use of modern, molecular and non-invasive fetal diagnostics. In contrast to other tests, the FastQ® RHD fetal kit types three exons of the RHD gene instead of only one exon. This allows for safe and targeted ...
Manufactured by:Yourgene Health based inManchester, UNITED KINGDOM
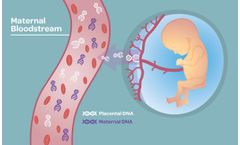
The IONA test is a non-invasive prenatal screening test to estimate the risk of a fetus having Trisomy 21, Trisomy 18 or Trisomy 13. The test can also be used to determine the sex of the ...
Manufactured by:ViennaLab Diagnostics GmbH based inVienna, AUSTRIA
A reliable tool to personalize therapy of lung cancer patients with acquired TKI-resistance mutation T790M in the EGFR ...
Manufactured by:Biocept based inSan Diego, CALIFORNIA (USA)
As healthy and diseased tissue interact with the bloodstream, they release both cellular and genetic material into the circulation. These materials are referred to as “cell-free” DNA (cfDNA), and circulating tumor cells (CTCs). Conventional blood collection tubes contain only anti-coagulants, designed to prevent blood ...
Manufactured by:Sysmex Corporation based inChuo-ku, JAPAN
In general, gene amplification (such as the RT-PCR method) reaction requires a process to prevent impurities in the sample from affecting the gene amplification reaction. Also, it takes more than one hour for the gene amplification reaction. Sysmex has developed the one-step measurement technology for cancer cell derived biomarkers (CK19mRNA) in the lymph node by optimizing composition of the ...
Manufactured by:AffinityDNA based inEl Paso, TEXAS (USA)
Our Non-Invasive Prenatal Paternity Test is a cutting-edge test that makes it possible for you to confirm if the man you suspect to be the father of your baby is the biological father. Available from the 9th week of pregnancy, starting from the first day of your last menstrual period, you can get the answer you need with a risk-free DNA test. The Certainty™ test, with court-admissible ...
Manufactured by:Yourgene Health based inManchester, UNITED KINGDOM
The Sage™ Prenatal Screen is a non-invasive prenatal screening solution to estimate the risk of a fetus having Trisomy 21, Trisomy 18, Trisomy 13, Rare Autosomal Aneuploidies (RAA), Sex Chromosome Aneuploidies (SCA) and the most clinically relevant ...