- Home
- Equipment
- turkmenistan
- medical device sterilization
Refine by
Medical Device Sterilization Equipment & Supplies In Turkmenistan
61 equipment items found
Manufactured by:MDS Medical Devices Sterilizers based inIvry le Temple, FRANCE
Worldwide preferred technology for low temperature sterilization of single use medical devices is Ethylen Oxide (EtO). This sterilization process is optimized by MDS to offer fully turnkey solution for medical device manufacturers and sterilization services providers. Our ...
Manufactured by:Nordion (Canada) Inc. based inOttawa, ONTARIO (CANADA)
The Parallel Row Pallet Irradiator provides the ideal solution for processing intact pallets of product, reducing material handling labour cost while maximizing throughput. Ideal for both phytosanitary applications and medical device sterilization, the Pallet Irradiator accommodates a variety of product densities and dose uniformity requirements. ...
Manufactured by:RSD Engineering Solutions SL based inCanovelles, SPAIN
We design, manufacture, control and qualify Ethylene Oxide Sterilizers (EO/ETO) for the sterilization of thermo sensitive products (sensitive to heat and humidity) like syringes, catheters, dialysis cartridges, plastic dressings, sutures, etc. We are Ethylene oxide sterilizers manufacturer, the perfect method for medical ...
Manufactured by:MDS Medical Devices Sterilizers based inIvry le Temple, FRANCE
Our V90 and Steam Pot steam sterilizers have been developed as an emergency solution: the quickest and easiest autoclave, respecting high steam international sterilization standards. It is mostly intended for field hospitals and healthcare centres, to sterilize surgical instruments and medical devices under ...
Manufactured by:Huons Medicare based inBusan, SOUTH KOREA
Purpose of use: sterilization of small spaces. Size (W*D*H): 500*20*495mm. weight: 19.5kg. voltage: 220V (60Hz) Sterilization of small spaces: Up to 150? ...
Manufactured by:Molteni Therapeutics s.r.l based inScandicci (FI), ITALY
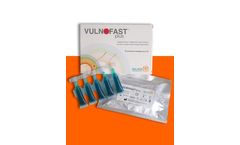
VULNOFAST® plus is an improved formulation of VULNOFAST® gel 0.3%. VULNOFAST® plus is a sterile medical device (CE0373) topically administered recommended as an adjuvant for the local treatment of skin lesions and ulcers through Photodynamic Therapy. The device is used in combination with a red light source ...
Manufactured by:DUK-IN based inSeoul, SOUTH KOREA
Class 1 medical device, E.O GAS Sterilization,Size: 1. 100ml, 2. Normal (200 ~ 400ml), Suture Free securement for VAC tube0, P.rotects from accidental tube removal, Painless for the patient(both securing and removal), Accurate inspection of puncture site through dressing window (Bleeding, Tube kink, etc), Convenient dressing, Easy ...
Manufactured by:JCM MED based inSuze-la-Rousse, FRANCE
IV cannulas with wing holder / cover, specially designed for one-handed insertion. IV cannulas with wing holder / cover, specially designed for one-handed insertion. The device carries small wings, specially designed for easy gripping and safe clamping to allow secure fixation to ...
Manufactured by:Molteni Therapeutics s.r.l based inScandicci (FI), ITALY
VULNOFAST® gel 0.3% is a sterile medical devices (CE0373) topically administered recommended as an adjuvant for the local treatment of skin lesions and ulcers through Photodynamic Therapy. The device is used in combination with a LED red light source at 630 ...
Manufactured by:Molteni Therapeutics s.r.l based inScandicci (FI), ITALY
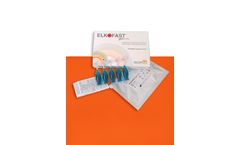
ELKOFAST® gel 0.3% is a non sterile medical device (CE0373) topically administered recommended as an adjuvant for the local treatment of Skin lesions and ulcers through Photodynamic Therapy. The device is used in combination with a LED red light source at 630 ...
Manufactured by:hawo GmbH based inObrigheim, GERMANY
The band sealers hpl 500 D and hpl 500 D-V are ideal for sealing all kinds of industrial products with thermoplastic polymer films (without contact pressure system) as well as compostable and biodegradable materials such as ORGANIXprotect. Packaging with the hpl 500 D/D-V offers maximum protection for the packaged item during transport and storage and complies with the strictest safety ...
Manufactured by:Advanced Sterilization Products (ASP) based inIrvine, CALIFORNIA (USA)
Easy-to-read process indicators to differentiate processed from unprocessed medical device packages to be sterilized in STERRAD® Sterilization Systems. SEALSURE® CI Tape and STERRAD™ CI Strips change from red to color (or lighter) indicated by comparator bar when exposed to hydrogen peroxide to differentiate between ...
Manufactured by:JETEMA, Co., Ltd. based inWonju-si, SOUTH KOREA
Product name : Graft/prosthesis, biomaterial; Product Authorization Number : 17-594; Storage : 2°C ~ 25°C (Do not freeze); Indication : Please refer to the instruction for use; [Single use / Sterile medical devices / Do not ...
Manufactured by:JETEMA, Co., Ltd. based inWonju-si, SOUTH KOREA
Product name : Graft/prosthesis, biomaterial; Product Authorization Number : 17-203; Storage : 2°C ~ 25°C (Do not freeze); Indication : Please refer to the instruction for use; [Single use / Sterile medical devices / Do not ...
Manufactured by:hawo GmbH based inObrigheim, GERMANY
The new heat sealer hm 950 DC-V NanoPak surpasses all its predecessors, offering smart operation, comprehensive digitisation, networkability and sustainability, reflecting the spirit of industry 4.0. The design of the NanoPak is based on the award-winning design of hawo’s Gereration Easy series. We have designed the devices of this product generation to meet the high standards, in terms of ...
Manufactured by:Sterimed based inCoulommiers, FRANCE
Ethypel Performance SP 63 gsm is a medical cellulose-based overall coated top web solution designed to provide a controlled and consistent sealing performance for optimized peel ability requirements when combined to films made of a PE seal layer (PA/PE ; PP/PE ; PET/PE, etc.). Ethypel Performance SP 63 gsm is made of a 60 gsm cohesive base sheeting coated with a waterbased 3 gsm adhesive layer ...
Manufactured by:Metrex Research, LLC. based inOrange, CALIFORNIA (USA)
Pre-cleaner used on medical devices prior to terminal sterilization or high-level disifection. Contains two protease enzymes that offer a broad cleaning action on a variety of protein soils. Inclusion of surfactants provides additional cleaning on carbohydrates, lipid and protein soils. ...
Manufactured by:Galbino Technology Inc. based inMarkham, ONTARIO (CANADA)
The Galbino ZW2211A series filter container has been specifically engineered for optimal performance in hospital sterile supply environments. It is constructed from high-quality materials that facilitate efficient heat conduction and exhibit excellent corrosion resistance while maintaining a lightweight profile. The series offers the convenience of either disposable or reusable filters to enhance ...
Manufactured by:EXPlasma - CUBE Instrument Inc. based inDaegein, SOUTH KOREA
Low temperature plasma sterilizers are sensitive to moisture and temperature differences. If there is moisture or a temperature difference the hydrogen peroxide used as a sterilant may be on the medical device. Preheating and moisture removal are very important. EXPIasma is a sterilization solution that can ...
Manufactured by:ASANUS Medizintechnik GmbH based inNeuhausen ob Eck, GERMANY
The ASANUS retractor covers STERI COVER have been proving their worth in everyday operating theatres for years - following recertification, they are now available with IIA approval in accordance with Annex II of Directive 93/42/EEC for medical devices. The sterile single use retractor covers from ASANUS offer an easy and quick application due to ...