- Home
- Equipment
- usa hawaii
- preclinical phase
Show results for
Refine by
Applications
- Medical cannabis solutions for regulatory pathway sector
- Medical cannabis solutions for mental health costs sector
- Clinical Immunology and Intensive Care Medicine for Haemophilia
- Clinical Immunology and Intensive Care Medicine for Immunoglobulins
- Clinical Immunology and Intensive Care Medicine for Antibody Deficiency
Preclinical Phase Equipment & Supplies In Usa Hawaii
28 equipment items found
by:AbelZeta Inc. based inRockville, MARYLAND (USA)
PRODUCT: C-CAR036. INDICATION: Solid Tumor. PLATFORM: CAR-T. PRECLINICAL: Claudin 18.2. IIT: -. PHASE 1/1B: -. PHASE 2: -. PARTNERS: ...
by:AbelZeta Inc. based inRockville, MARYLAND (USA)
PRODUCT: C-CAR168. INDICATION: Autoimmune. PLATFORM: CAR-T. PRECLINICAL: CD20 / BCMA. IIT: -. PHASE 1/1B: -. PHASE 2: -. PARTNERS: ...
by:AbelZeta Inc. based inRockville, MARYLAND (USA)
PRODUCT: C-CAR031. INDICATION: HCC. PLATFORM: CAR-T. PRECLINICAL: GPC3. IIT: -. PHASE 1/1B: -. PHASE 2: -. PARTNERS: ...
by:AbelZeta Inc. based inRockville, MARYLAND (USA)
PRODUCT: C-CAR066. INDICATION: r/r B-NHL. PLATFORM: CAR-T. PRECLINICAL: CD20. IIT²: - PHASE 1/1B: - PHASE 2: - PARTNERS: Johnson & ...
Manufactured by:Gesynta Pharma AB based inStockholm, SWEDEN
The drug candidate GS-659, which is sprung from Gesynta Pharma’s drug development platform, is currently in preclinical development phase. Project updates will be announced in due ...
Manufactured by:Biotest AG based inDreieich, GERMANY
At the ISTH 2020 Biotest presented its novel recombinant Factor VIII molecules overcoming the major challenges of current Factor VIII replacement therapy by combining a significantly extended half-life, low immunogenicity and the possibility of either subcutaneous or intravenous ...
Manufactured by:iMouse GmbH based inBerlin, GERMANY
Its intelligent Vision AI and machine learning platform aids scientists in refining the prediction of new drug candidates, enhancing translation from preclinical to clinical phases while minimizing failure ...
Manufactured by:EndoLogic LLC based inClifton, NEW JERSEY (USA)
Renzapride is best suited as a treatment of Diabetic Gastroparesis, a condition with a significant unmet treatment options. In the numerous trials that have been done using Renzapride more than 5000 patients have been treated with Renzapride. An extensive cardiac safety evaluation under the FDA mandated Thorough QT trial has shown that Renzapride does not have any cardiac safety ...
Manufactured by:Magellan based inBox Hill North, AUSTRALIA
Our product MAG200 has successfully completed Phase I/II of a clinical trials on the use of allogeneic (donor) off-the-shelf stem cell therapy for OA. The pivotal Phase III trial is planned to commence 2023. ...
Manufactured by:CanaQuest Medical Corp. based inMississauga, ONTARIO (CANADA)
A variety of things are evaluated through clinical trials, including: Effectiveness of medications, minimum and ultimate dose, medication combinations. Based on positive results from the preclinical research, we are ready to move forward with a clinical trial to see how well it works in humans. Clinical trials happen in several phases during which different ...
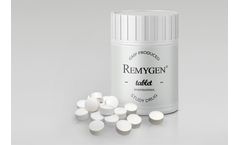
Manufactured by:Diamyd Medical AB based inStockholm, SWEDEN
Remygen® is a controlled release tablet consisting of the active pharmaceutical ingredient GABA. The GABA substance is chemically synthesized in a well-controlled GMP process together with a global contract manufacturer. The manufacturing process is scalable and pediatric formulations with the same properties can be designed. ...
Manufactured by:Cabaletta Bio, Inc. based inPhiladelphia, PENNSYLVANIA (USA)
PLA2R-CAART is being developed to treat patients with PLA2R-associated membranous nephropathy (MN), a B cell-mediated autoimmune disease that affects the kidneys. B cells produce autoantibodies, which are believed to form immune complexes that are deposited at the glomerular basement membrane, causing damage of the filtration barrier, and leading to proteinuria. Over time, ...
Manufactured by:SiO2 Materials Science based inAuburn, ALASKA (USA)
We provide a common drug contact surface starting at the research and pre-clinical drug development phases, through clinical trials, and on through to commercial ...
Manufactured by:TVAX Biomedical, Inc. based inOlathe, KANSAS (USA)
The drug candidate has received Fast Track Designation by the US Food and Drug Administration (FDA) to test TVI-Brain-1 in a Phase 2b clinical study for glioblastoma multiforme (GBM). The Fast Track Designation is supported by positive Phase 2 clinical data, as well as extensive preclinical and Phase 1 safety ...
Manufactured by:TVAX Biomedical, Inc. based inOlathe, KANSAS (USA)
TVAX®’s second candidate (TVI-Kidney-1) is being evaluated for the treatment of kidney cancer and targets stage 4 renal cell ...
by:Alume Biosciences, Inc. based inCA, CALIFORNIA (USA)
Loss of nerve function can be catastrophic. As many as 80% of prostate surgeries result in incontinence or erectile dysfunction because of nerve damage, and as many as 25% of head and neck surgeries damage the facial nerves. The mission of Alume Biosciences is to make our proprietary nerve illumination technology available for surgeons so that patients everywhere can benefit. Learn how you can ...
Manufactured by:SiO2 Materials Science based inAuburn, ALASKA (USA)
We provide a common drug contact surface starting at the research and pre-clinical drug development phases, through clinical trials, and on through to commercial ...
Manufactured by:Diamyd Medical AB based inStockholm, SWEDEN
Diamyd® is a suspension for injection containing the active pharmaceutical ingredient GAD65 mixed with the vaccine adjuvant Alhydrogel (alum). The human recombinant protein GAD65 is manufactured in insect cells in a well-controlled GMP process. The manufacturing process is developed for late phase clinical use. A new manufacturing facility is being set up by Diamyd Medical in Umeå, ...
Manufactured by:Ocean Biomedical based inProvidence, RHODE ISLAND (USA)
New therapeutic antibodies for effective treatment of NSCLC and other disorders. We are moving towards Phase 1 clinical trials of patented antibodies for the treatment of NSCLC. Our lead program, OCX-253 was chosen because it stimulates the B-Raf protooncogene while inhibiting apoptosis, NK cell accumulation and activation, LIM kinase 2, p-cofilin, the tumor suppressor PTEN, and p53. In addition, ...
Manufactured by:ARANZ Medical Limited based inChristchurch, NEW ZEALAND
A proven wound assessment and management solution for both clinical practice and clinical research. ...