- Home
- Equipment
- usa rhode island
- embolic prevention
Show results for
Refine by
Embolic Prevention Equipment & Supplies In Usa Rhode Island
7 equipment items found
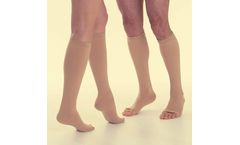
Manufactured by:Calze G.T. S.r.l. based inAsola, ITALY
Relaxsan Medicale is a line of state-of-the-art compression stockings made of high-quality and fine yarns which guarantee strength and durability. Relaxsan Medicale Stockings come in a variety of sizes, lengths, compression levels, and materials in order to provide our customers with a customized solution to the treatment for venous ...
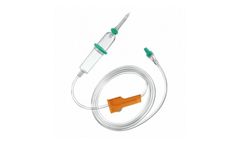
Manufactured by:CP Medical based inNorcross, GEORGIA (US) (USA)
Intrafix SafeSet II Tube Length 90” PVC, DEHP free. 100 ...
Manufactured by:Abbott Laboratories based inAbbott Park, ILLINOIS (USA)
Backed by a 15-year track record of performance in the U.S.,1 the Amplatzer Duct Occluder can accommodate large PDAs with a single device—which can aid in minimizing the complexity of the ...
Manufactured by:Adient Medical, Inc. based inPearland, TEXAS (USA)
Adient Medical is leading the development of an absorbable vascular filter for the prevention of pulmonary embolism (PE), a leading cause of death in the US claiming the lives of over 100,000 Americans annually - more than breast cancer, AIDS, and traffic fatalities combined. ...
Manufactured by:Silk Road Medical, Inc. based inSunnyvale, CALIFORNIA (USA)
The ENROUTE Transcarotid Neuroprotection System provides direct carotid access with robust flow reversal prior to crossing the lesion for embolic protection during angioplasty and stenting. The device removes micro and macro emboli throughout the intervention for CEA-like neuroprotection in a less invasive ...
Manufactured by:Invamed based inChampionsGate, FLORIDA (USA)
The system requires the use of a specialized system that creates a Rotational vortex effect that prevents embolism and macarates thrombus with TPA while preventing distal ...
Manufactured by:Adient Medical, Inc. based inPearland, TEXAS (USA)
Although conventional metal IVC filters can reduce the incidence of PE to 1-2%, many are fraught with mounting complications as they remain in the body for prolonged duration. Most IVC filters are placed with the intention to be retrieved within weeks as recommended by the FDA. However, only a small percentage of patients (4-7% in statewide studies) return for the subsequent retrieval procedure. ...