Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Covid 19 Rt Pcr Detection Equipment & Supplies In Usa
35 equipment items found
Manufactured by:Genabio Diagnostics Inc. based inBedford, MASSACHUSETTS (USA)
cIntended Use: Fosun COVID-19 RT-PCR Detection Kit is a real-time RT-PCR test intended for the qualitative detection of nucleic acid from the SARS-CoV-2 in upper and lower respiratory specimens (such as anterior nasal swabs, mid-turbinate nasal swabs, ...
Manufactured by:Heinz Herenz Medizinalbedarf GmbH based inHamburg, GERMANY
For detection of oxygen saturation (SPO2) and pulse rate through finger, dependable oxygen level and pulse rate, display SpO2, PR, pulse bar, dual color OLED display, 2 AAA alkaline batteries. Accommodates widest range of finger sizes from pediatric to adult. SPO2 Measuring range: 35% - 99%, PR Measuring range: 30bpm – ...
Manufactured by:EirGenix, Inc based inNew Taipei City, TAIWAN
It can detect COVID-19 and get result in 10 minute! ...
Manufactured by:NanoEnTek, Inc. based inGuro-gu, SOUTH KOREA
The En-swer™ COVID-19 RT-PCR Kit detects E, N, and RNaseP genes of SARS-CoV-2 qualitatively through real-time reverse-transcription polymerase chain reaction. It can be used for rapid detection and outbreak control of COVID-19. ...
Manufactured by:Pathnostics based inIrvine, CALIFORNIA (USA)
The Pathnostics COVID-19 PCR Test detects the presence of SARS-CoV-2, the virus that causes COVID-19. The Pathnostics COVID-19 PCR Test is not impacted by the emerging B.1.1.529 (Omicron variant), enabling accurate test ...
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
The MaximBio ClearDetect™ COVID-19 Antigen Home Test is an antigen test that allows you to detect proteins from the virus that causes COVID-19. Made easy - An over-the-counter, no prescription needed nasal swab test you can do anywhere & anytime. Know your COVID-19 status in as little as 15 minutes! The MaximBio ClearDetect™ COVID-19 Antigen Home Test is only for use under the ...
Manufactured by:OPTOLANE Technologies Inc. based inSeongnam-si, SOUTH KOREA
The Genoplexor™ COVID-19 Detection Kit is an in vitro diagnostic kit designed for real-time reverse transcription polymerase chain reaction test intended for the qualitative detection of SARS-CoV-2 (RdRP gene, S gene) RNA in human samples such as human upper respiratory (oropharyngeal swab and nasopharyngeal swab) from individuals with suspected or confirmed respiratory ...
Manufactured by:LabTurbo Biotech Corporation based inTaipei City, TAIWAN
LabTurbo™ AIO COVID-19 RNA Testing Kit is a multiplex real-time reverse transcription polymerase chain reaction, (RT-PCR) diagnostic product for SARS-CoV-2 RNA (COVID-19) detection. The multiplex reaction detects COVID-19 ...
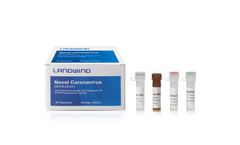
Manufactured by:Shenzhen Landwind Medical Co., Ltd based inShenzhen, CHINA
Antigen detection can be used for early disease screening, the experiment is simple and rapid, suitable for large-scale detection, and can be used as a supplementary program for nucleic acid detection ...
Manufactured by:GENCURIX based inGuro-gu, SOUTH KOREA
What is this test? GenePro COVID-19 Detection Test is developed based on “WHO interim guidance for laboratory testing for 2019 novel coronavirus (SARS-CoV-2) in humans” by WHO (World Health Organization), and qualitatively detects RdRP gene / E gene simultaneously. RdRP gene has specific sequence from ...
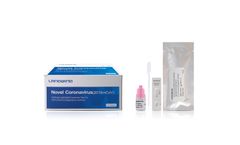
Manufactured by:Shenzhen Landwind Medical Co., Ltd based inShenzhen, CHINA
The genetic material of COVID-19 is RNA. The principle of the RT-PCR method is the specific match between the primers and probes and the specific region of COVID-19 RNA. Once the RNA of the virus is detected, the result will be "positive". In terms of Methodology, the nucleic ...
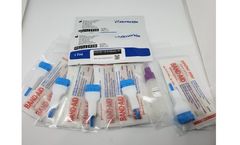
Manufactured by:Shenzhen Landwind Medical Co., Ltd based inShenzhen, CHINA
When the human body contacts with foreign antigens, the first antibody produced is IgM, which is secreted directly by the receptor on the surface of B cells. The B cells that produce IgM enter the lymph nodes, receive stimulation from T cells and antigen-presenting cells in the center of occurrence, further mature, differentiate into plasma cells, and produce large amount of IgG. ...
Manufactured by:America Diagnostics, Inc. based inSan Diego, CA 92126 USA, CALIFORNIA (USA)
This product is for in vitro diagnostic use, following guidance from the FDA for Emergency Use Authorizations of tests submitted for approval on March 16, 2020. This test has not been reviewed by the FDA and results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection ...
Manufactured by:America Diagnostics, Inc. based inSan Diego, CA 92126 USA, CALIFORNIA (USA)
This product is for in vitro diagnostic use, following guidance from the FDA for Emergency Use Authorizations of tests submitted for approval on March 16, 2020. This test has not been reviewed by the FDA and results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection ...
Manufactured by:Access Bio based inSomerset, NEW JERSEY (USA)
Self Test for SARS-CoV-2 Antigen Detection. The CareStart™ COVID-19 Antigen Home Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigens from SARS-CoV-2. Fast and easy to self-test at anywhere. Easy to interpret the results using mobile application. Qualitatively detect the SARS-CoV-2 nucleocapsid protein. Use for nasal swab specimen. Fast ...
Manufactured by:Convergent Technologies GmbH & Co. KG based inCoelbe, GERMANY
Convergys® POC RT-PCR COVID-19 Detection Kit.m for use with the Convergys® POC RT-PCR Nucleic Acid Detection System. The COVID-19 respiratory disease is caused by infection with the novel coronavirus SARS-CoV-2. The ...
Manufactured by:Wuhan HealthCare Biotechnology Co., Ltd. based inWuhan, CHINA
For qualitative detection of the nucleocapsid protein antigen of SARS-CoV-2 in nasopharyngeal and nasal swab, and saliva specimens from individuals and is aid in rapid diagnosis of patients with suspected SARS-CoV-2 ...
Manufactured by:Molbio Diagnostics Pvt. Ltd. based inVerna, INDIA
Intended Use: A lyophilized, ready-to-use, open format, duplex Real Time Reverse Transcription Polymerase Chain Reaction (RT PCR) test for the qualitative detection of SARS CoV-2 RNA in human oropharyngeal and nasopharyngeal swabs and aids in the diagnosis of COVID-19. The test detects the E and ...
Manufactured by:Nirmidas Biotech, Inc. based inPalo Alto, CALIFORNIA (USA)
The MidaSpot COVID-19 Antibody Combo Detection Kit is a lateral flow immunoassay intended for the qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2 in human serum, plasma (EDTA or lithium heparin), and capillary whole blood (POC fingerstick) . Nirmidas has obtained FDA EUA for this test.isting fluorescence based microplate ...
Manufactured by:Wuhan HealthCare Biotechnology Co., Ltd. based inWuhan, CHINA
For qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2 in serum, plasma (EDTA, sodium citrate and lithium heparin) or fingerstick whole blood, venous whole blood specimens from patients suspected of COVID-19 ...