Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Femoral Component Equipment & Supplies In Usa
44 equipment items found
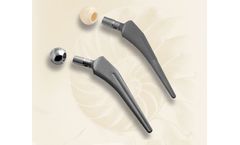
Manufactured by:PBS Velká Bíteš, a.s. based inVelká Bíteš, CZECH REPUBLIC
První brněnská strojírna is a specialized manufacturer of castings made of superalloys based on Co-Cr-Mo. These also include castings for the healthcare industry. Due to their purpose of use, extremely high demands are placed on these ...
Manufactured by:Consensus Orthopedics based inEl Dorado Hills, CALIFORNIA (USA)
The Consensus Revision Knee System provides a seamless adjustment of our consensus knee system which means it’s as if nothing changed in the first place. The Consensus Revision Knee System allows for seamless primary to revision system compatibility. Multiple augment options help restore natural joint line and interchangeable stem lengths aid in accurate component fit and ...
Manufactured by:Consensus Orthopedics based inEl Dorado Hills, CALIFORNIA (USA)
The consensus knee System has expanded its implant offerings to this mobile bearing design to reduce wear and provide surgeons with a dual articulation and inserts are designed to be compatible with Consensus Knee femoral and patellar ...
Manufactured by:Consensus Orthopedics based inEl Dorado Hills, CALIFORNIA (USA)
The Consensus Knee System has been developed on a foundation of established design principles, proven clinical experience and intelligent engineering. The result is a Knee System incorporating state-of-the-art features providing excellent performance – predictably and ...
Manufactured by:CORENTEC Co., Ltd. based inCheonan-si, SOUTH KOREA
Femoral Component: Femoral Component is a typical double-tapered wedge stem, designed to suit the anatomical structure in the marrow cavity. The section is rectangular-shaped, allowing a strong initial press-fit fixation in the femoral marrow cavity. ...
Manufactured by:CORENTEC Co., Ltd. based inCheonan-si, SOUTH KOREA
Femoral Component: Single axis design for articular stability with flexion and improving range of motion. Lateralized anterior trochlea groove for optimal patella tracking. Spherical articular surface for reducing contact stress and improving ...
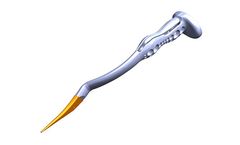
Manufactured by:Onkos Surgical based inParsippany, NEW JERSEY (USA)
The ELEOS proximal femoral system consists of a femoral component, optional midsection, and stem. The femoral component is compatible with various femoral heads and bipolar options. ...
by:INNOPROOF GmbH based inRostock, GERMANY
We offer you a characterization of the restriction of the range of motion of knee endoprostheses regarding the translational and rotational degrees of freedom according to ASTM F1223. For this purpose, either the tibia or the femoral component is firmly clamped to the test device while the other side remains movable. The stability of your individual knee ...
Manufactured by:CORENTEC Co., Ltd. based inCheonan-si, SOUTH KOREA
Increased Flexion, Increased Stability. Femoral Component: Single axis design for articular stability with flexion and improving range of motion. Anterior flange shape and dimension for the optimal patella tracking. Intercondylar cam design of PS type for reducing the risk of dislocation in deep flexion. ...
Manufactured by:United Orthopedic Corporation (UOC) USA Inc. based inIrvine, CALIFORNIA (USA)
The U2 MB (Mobile Bearing) Knee is a rotating tibial platform design intended to facilitate prosthetic alignment and lower shear force on the bone-implant interface. The U2 MB Knee is compatible with both U2 fixed bearing Posterior Stabilized (PS) and Cruciate Retaining (CR) femoral ...
Manufactured by:Biotech GmbH based inRheinbrohl, GERMANY
The concept of this femoral component relies on a close prosthests-bone contact in both medial and lateral zones The stem geometry shows a proximal lo distal 3 degree taper providing a greater femoral canal fill, improving rotational stability and transferring load in the proximal cakar region to restore near normal stress distribution Proximal ...
by:INNOPROOF GmbH based inRostock, GERMANY
The tests according to ISO 7206-4 and -6 refer to the shaft or neck area of the femoral component of hip endoprostheses. The fatigue strength of the hip stem is determined under a given sinusoidal load and over a defined number of cycles. The clamping as well as the applied loads differ according to the design of the hip stem, classified as a short, standard or ...
Manufactured by:Surgival based inPaterna, SPAIN
Notably, it accommodates large bone defects that exacerbate these instabilities. The system requires cemented application of its femoral components and tibial trays, while recommending non-cement use for straight stems and offset designs. This ensures optimized compatibility and stability during surgical procedures. Additionally, components such ...
Manufactured by:Tecomet, Inc. based inWilmington, MASSACHUSETTS (USA)
With unmatched design support and state-of-the-art forging, casting, and finishing capabilities, Tecomet is your end-to-end solution for high-precision femoral ...
Manufactured by:MedSpark, LLC based inSan Luis Obispo, CALIFORNIA (USA)
Such joint replacement orthopedic surgery is generally conducted to relieve arthritis pain or fix severe physical joint damage such as a hip fracture. During this procedure, the femoral head is typically resected and the proximal femoral canal is broached and/or milled to accept the femoral component with attached prosthetic ...
Manufactured by:MedSpark, LLC based inSan Luis Obispo, CALIFORNIA (USA)
Total Knee Arthroplasty (replacement) or TKA is a surgical procedure in which the knee joint is replaced by a prosthetic implant. During this procedure, the distal end of the femur must be prepared to accept the femoral component of the replacement joint construct. Following the initial resection, a saw guide is temporarily pinned on the end of the femur and a ...
Manufactured by:Surgival based inPaterna, SPAIN
Developed by Surgival, this system integrates a balance of resistance, mobility, fixation, and durability, addressing both cemented and cementless applications. The prosthesis is engineered with several components, including the femoral component, tibial insert, tibial tray, and supplements, ensuring versatility and effectiveness in diverse ...
Manufactured by:MedSpark, LLC based inSan Luis Obispo, CALIFORNIA (USA)
Total Knee Arthroplasty (replacement) or TKA is a surgical procedure in which the knee joint is replaced by a prosthetic implant. During this procedure, the distal end of the femur must be prepared to accept the femoral component of the implant. During a typical surgical procedure, a sagittal saw (see pictures) is utilized by the surgeon to shape the bone. In ...
Manufactured by:MedSpark, LLC based inSan Luis Obispo, CALIFORNIA (USA)
Such joint replacement orthopedic surgery is generally conducted to relieve arthritis pain or fix severe physical joint damage such as a hip fracture. During this procedure, the femoral head is typically resected and the proximal femoral canal is broached and/or milled to accept the femoral component with attached prosthetic ...
Manufactured by:Kinamed Incorporated based inCamarillo, CALIFORNIA (USA)
Designed specifically for the small but challenging group of patients with isolated, end-stage patello-femoral disease, the KineMatch PFR (Patello-Femoral Replacement) offers a uniquely effective and conservative resurfacing solution. Because the device is precisely pre-fitted to the patient’s anatomy using CT (Computed Tomography) data, no resection of ...