EOS MEDICAL SOLUTIONS (EOSMED)
- Home
- Companies
- EOS MEDICAL SOLUTIONS (EOSMED)
- Products
- EOS - Biongraft Putty

EOS - Biongraft Putty
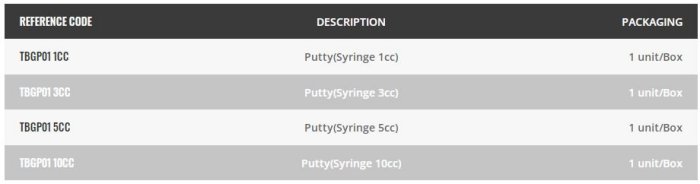
Biongraft is an injectable and formable paste bone graft based on hydrogel and Beta-tricalcium phosphate (β-TCP), including ZrO2 nanoparticles for antibacterial e?cacy. Biongraft is a safe and fully biocompatible material which is designed to act as an osteoconductive sca?old to support the ingrowth and fusion of adjacent viable bone when placed in an osseous environment .It also provides a bone void ?ller that resorbs and is replaced with bone during the healing process. Requires no mixing. Pre-loaded in a convenient applicator, syringe ready for direct placement into the defect. Easily molded by hand as needed.
Most popular related searches
bone substitutes
healing process
bone substitute
knee revision
disease transmission
bone material
surgery product
orthopedic spine
surgery room
synthetic bone
- Ready to Use : Pre-loaded in a convenient applicator, syringe ready for direct placement in the defect. Easily molded by hand as needed. Requires no mixing.
- %100 synthetic : Contains no tissue of human or animal origin therefore carries no risk of disease transmission.
- Osteoconductive : Provides a scaffold for new bone. Highly interconnected porosity with excellent mechanical resistance.
- Biodegradable : With its optimized porous structure and chemical composition, our products are suitable for the continuous remodeling cycle of healthy bone. β-TCP resorbs over time and being replaced with bone during the healing process.
Biongraft is intended for use in non-load bearing defects in:
- Osteotomies
- Hip and Knee Revision Surgery
- Fractures with loss of bone material
- Acetabular reconstruction
- Metaphysial fractures
- Extremity fractures
- Filling cages in spinal surgery
- Step 1: Open the blister in sterile area. Remove the cap of the injector in sterile ?eld just ten minutes before the implantation. Biongraft is supplied as a paste in the injector, ready for use.
- Step 2: Fill the defect area completely or shape bone substitutes according to the condition of the defect area. It should come into direct contact with all surfaces on the defect.
- Step 3: Secure the surgical site after implanting in order to prevent motion, leakage or implant migration. When excess ?uid is present in the surgical ?eld, the surgeon may use proper measures (i.e. catheterization, suction and application of bone wax) to reduce bleeding.