

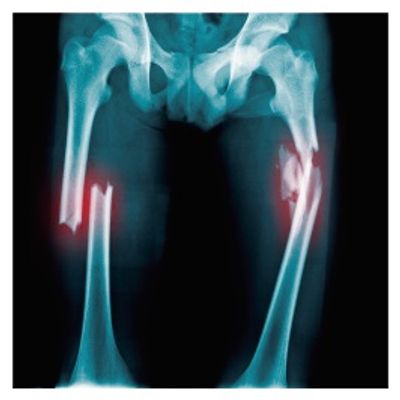
Bonecure Platform
ASC pre-differentiated to osteoblasts in Histobone scaffold (engineered synthetic bone substitute) for the treatment of bone defects caused by pseudoarthrosis, infections, resections, trauma).
Regenerative capabilities of cells.
Guided osseous re-modelling process due to scaffold properties.
Excellent integration of newly formed tissue with surrounding tissue.
Adequate revascularisation.
Clinical Phase II ongoing Q2 2018 with allogenic product.
Licensing & co-development agreement with Salvat.
2017: 6 patients treated with Bonecure autologous product and medical discharged as bone consolidation has been achieved.
Recruitment of phase II was completed in 2019.
2018-2020: evaluation of the clinical trial with 6 patients treated with allogeneic Bonecure.
