Refine by
Applications
- Clinical Immunology and Intensive Care Medicine for Haemophilia
- Clinical Immunology and Intensive Care Medicine for Immunoglobulins
- Clinical Immunology and Intensive Care Medicine for Antibody Deficiency
- Drug Dolution for Liver Metastases
- Drug Dolution for Gastric Cancer
- Microbiome Therapeutics for Clinical Trials
- Microbiome Therapeutics for Patients and Physicians
- Medical solutions for MiniTC and mini-stem system sector
- Solution for Ultrafast Laser Spectroscopy / Imaging
- Solution for High-Speed 4D Imaging
- The mat (t) isse bioprothesis: a bio-resorbable tec (tissue engineering chamber)
- Nuclear Medicine for Therapy
- Nuclear Medicine for Theragnostics
Clinical Development Equipment & Supplies
206 equipment items found
Manufactured by:Frontier Biotechnologies Inc. based inNanjing, CHINA
FB2001 (generic name: Bofutrelvir) is a SARS-CoV-2 3CL protease inhibitor under clinical development for hospitalized patients as well as for post-exposure prophylaxis of Covid-19. It is a peptidomimetic compound targeting 3CL-protease based on three-dimensional crystal structure of the protease. Preclinical data has shown it has potent activities in vitro and in ...
Manufactured by:AB2 Bio Ltd. based inLausanne, SWITZERLAND
Macrophage Activation Syndrome. Macrophage Activation Sysdrome (MAS) is a very severe complication of rheumatic diseases. Mortality ranges between 10-30% for patients with MAS and there is no registered therapy. MAS occurs frequently in systemic Juvenile Idiopathic Arthritis (soJIA) and in Adult onset Still’s Disease (AOSD) patients. It is characterized by severe systemic inflammation with ...
Manufactured by:AB2 Bio Ltd. based inLausanne, SWITZERLAND
People with HLH usually develop symptoms within the first months or years of life. Symptoms may include fever, enlarged liver or spleen, cytopenia (decreased number of blood cells), and neurological abnormalities. All forms of HLH, including cases treated adequately, may have a high mortality rate. The long-term outlook (prognosis) of familial forms without treatment is poor, with a median ...
by:Vivozon Inc. based inYongin-si, SOUTH KOREA
A randomized, double-blind, placebo-controlled, single and repeated dose, escalation clinical trial to evaluate the safety, tolerability, and pharmacokinetics of VVZ-149 injection in healthy adult ...
by:Vivozon Inc. based inYongin-si, SOUTH KOREA
Single-dose, escalation clinical trial to evaluate the safety and pharmacokinetics of VVZ-149 injection in healthy elderly ...
by:Vivozon Inc. based inYongin-si, SOUTH KOREA
A randomized, double-blind, parallel-group, placebo-controlled clinical trial to evaluate the analgesic efficacy and safety of VVZ-149 Injection in patients who have pain after laparoscopic gastrectomy in early gastric cancer ...
by:Vivozon Inc. based inYongin-si, SOUTH KOREA
A Randomized, double-blind, parallel group, placebo-controlled study to evaluate the analgesic efficacy and safety of VVZ-149 injections for post-operative pain following laparoscopic colorectal ...
by:Vivozon Inc. based inYongin-si, SOUTH KOREA
A randomized, double-blind, parallel-group, placebo-controlled clinical trial to evaluate the analgesic efficacy and safety of VVZ-149 Injection in patients who have pain after laparoscopic and robotic-laparoscopic ...
by:Vivozon Inc. based inYongin-si, SOUTH KOREA
A multicenter, randomized, double-blind, parallel-group, placebo-controlled phase 3 clinical trial to evaluate the analgesic efficacy and safety of VVZ-149 injection in patients who have pain after laparoscopic ...
by:Vivozon Inc. based inYongin-si, SOUTH KOREA
A randomized, double-blind, parallel group, placebo-controlled clinical trial to evaluate the analgesic efficacy and safety of VVZ-149 Injection in patients who have pain after artificial hip replacement ...
by:Vivozon Inc. based inYongin-si, SOUTH KOREA
Analgesic efficacy and safety of VVZ-149 injections for post-operative pain following laparoscopic colorectal surgery: a randomized, double-blind, parallel group, placebo-controlled, dose finding ...
Manufactured by:Ariana Pharma based inParis, FRANCE
Ariana® has consolidated multiple, publicly available administrative healthcare admissions databases into a locally searchable observational health data resource of patient hospitalization, outpatient and other episodes, comprising in excess of 6B records. ...
Manufactured by:Alzinova AB based inMölndal, SWEDEN
ALZ-101 is a vaccine that stimulates the production of antibodies against the toxic Aβ oligomers. A first-in-human study in patients with early Alzheimer’s disease was initiated during third quarter of ...
Manufactured by:EsoCap AG based inBasel, SWITZERLAND
EsoCap owns a unique drug delivery platform allowing the topical application of drug substances for targeted and long-lasting treatment of diseases of the upper gastrointestinal tract for the first ...
Manufactured by:HepaRegeniX GmbH based inTübingen, GERMANY
HepaRegeniX has started to progress the clinical development of its proprietary candidates e.g. ...
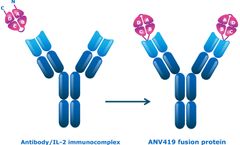
Manufactured by:Anaveon AG based inBasel, SWITZERLAND
The culmination of our research is ANV419, which is in clinical development. It is a rationally engineered fusion protein that is a stable, antibody-like molecule. ...
Manufactured by:Apyx Medical Corporation based inClearwater, FLORIDA (USA)
Renuvion cosmetic technology is the result of extensive scientific research and clinical development. Because of its proprietary balance of helium and RF energy, Renuvion has been described as ideal for medical procedures where precision and control are key to an effective ...
Manufactured by:CytomX Therapeutics, Inc. based inSouth San Francisco, CALIFORNIA (USA)
CytomX and Amgen are developing CX-904, a T-cell engaging bispecific Probody candidate against Epidermal Growth Factor Receptor (EGFR) and CD3. The drug candidate is advancing towards IND-enabling studies. CytomX is responsible for the IND filing, targeted for late 2021, and for early clinical development with Amgen leading later stage ...
Manufactured by:Creative Peptides based inNew York, NEW YORK (USA)
Abaloparatide is a parathyroid hormone-related protein (PTHrP) analog drug in clinical development for treating osteoporosis. Like the related drug teriparatide, and unlike bisphosphonates, it is an anabolic (i.e., bone growing) agent.https://www.creative-peptides.com/product/abaloparatide-item-10-101-172-33658.html ...
by:STALICLA based inGeneve, SWITZERLAND
DDU validates ASD phenotype groups and drug candidates through observational studies and investigational trials. DDU combines expertise in operations, medicine, drug and clinical development, to bring STALICLA’s clinical-stage treatment options closer to ...