Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Applications
- Clinical Immunology and Intensive Care Medicine for Haemophilia
- Clinical Immunology and Intensive Care Medicine for Immunoglobulins
- Clinical Immunology and Intensive Care Medicine for Antibody Deficiency
- Drug Dolution for Liver Metastases
- Drug Dolution for Gastric Cancer
- The mat (t) isse bioprothesis: a bio-resorbable tec (tissue engineering chamber)
- Nuclear Medicine for Therapy
- Nuclear Medicine for Theragnostics
Clinical Development Equipment Supplied In Europe
88 equipment items found
Manufactured by:AB2 Bio Ltd. based inLausanne, SWITZERLAND
Macrophage Activation Syndrome. Macrophage Activation Sysdrome (MAS) is a very severe complication of rheumatic diseases. Mortality ranges between 10-30% for patients with MAS and there is no registered therapy. MAS occurs frequently in systemic Juvenile Idiopathic Arthritis (soJIA) and in Adult onset Still’s Disease (AOSD) patients. It is characterized by severe systemic inflammation with ...
Manufactured by:AB2 Bio Ltd. based inLausanne, SWITZERLAND
People with HLH usually develop symptoms within the first months or years of life. Symptoms may include fever, enlarged liver or spleen, cytopenia (decreased number of blood cells), and neurological abnormalities. All forms of HLH, including cases treated adequately, may have a high mortality rate. The long-term outlook (prognosis) of familial forms without treatment is poor, with a median ...
Manufactured by:Ariana Pharma based inParis, FRANCE
Ariana® has consolidated multiple, publicly available administrative healthcare admissions databases into a locally searchable observational health data resource of patient hospitalization, outpatient and other episodes, comprising in excess of 6B records. ...
Manufactured by:Alzinova AB based inMölndal, SWEDEN
ALZ-101 is a vaccine that stimulates the production of antibodies against the toxic Aβ oligomers. A first-in-human study in patients with early Alzheimer’s disease was initiated during third quarter of ...
Manufactured by:EsoCap AG based inBasel, SWITZERLAND
EsoCap owns a unique drug delivery platform allowing the topical application of drug substances for targeted and long-lasting treatment of diseases of the upper gastrointestinal tract for the first ...
Manufactured by:HepaRegeniX GmbH based inTübingen, GERMANY
HepaRegeniX has started to progress the clinical development of its proprietary candidates e.g. ...
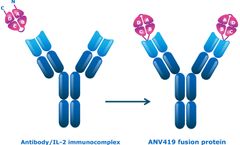
Manufactured by:Anaveon AG based inBasel, SWITZERLAND
The culmination of our research is ANV419, which is in clinical development. It is a rationally engineered fusion protein that is a stable, antibody-like molecule. ...
by:STALICLA based inGeneve, SWITZERLAND
DDU validates ASD phenotype groups and drug candidates through observational studies and investigational trials. DDU combines expertise in operations, medicine, drug and clinical development, to bring STALICLA’s clinical-stage treatment options closer to ...
Manufactured by:Genenta Science based inMilano, ITALY
We believe that LVVs are the first choice for ex-vivo gene therapy in humans because they can efficiently transduce HSPCs with potentially large transgenes that will allow us to expand therapeutic options to a multitude of payloads. Most importantly, there is an abundance of safety data generated using these vectors to develop novel medicines currently in ...
Manufactured by:3B Pharmaceuticals GmbH based inBerlin, GERMANY
3B Pharmaceuticals have built a drug discovery platform extending from hit identification to early clinical development. Several drug candidates developed through our platform already have been successfully administered to humans. ...
Manufactured by:Catalent, Inc based inSomerset, NEW JERSEY (USA)
Antibody-drug conjugates (ADCs) combine the targeted binding specificity and pharmacological advantages of monoclonal antibodies with the potency advantages of small molecule chemotherapy agents via conjugation with chemical linkers. With 10 approved products and a large and growing list of programs in clinical and pre-clinical development, ADCs ...
Manufactured by:Biotest AG based inDreieich, GERMANY
Trimodulin mediates its mode of action via three potential mechanisms which are; opsonization of causal pathogens, neutralizing of microbial pathogens and their virulence factors (endo- and exotoxins) and targeting the host inflammatory response (anti-inflammatory properties). Currently, trimodulin is under clinical development for severe community-acquired ...
Manufactured by:Gesynta Pharma AB based inStockholm, SWEDEN
GS-248 is Gesynta Pharma’s most advanced drug candidate, currently in clinical development as a potential treatment of systemic sclerosis. Recent clinical phase I data in healthy individuals show that GS-248 potently inhibits the production of PGE2 in a tolerable and safe manner, while increasing the observed levels of protective and ...
Manufactured by:MorphoSys AG based inPlanegg, GERMANY
Preclinical studies and translational insights from our first-in-human study of pelabresib led us to prioritize the clinical development of pelabresib in ...
Manufactured by:TC BioPharm based inHolytown, UNITED KINGDOM
TC BioPharm’s cell banks have been developed as a resource for clinical development of cost-effective, safe and efficacious therapeutic treatments and ultimately, the ability to treat more patients. These T cell banks provide TC BioPharm with core technology to develop a deep portfolio of next-generation CAR-T products ...
Manufactured by:ViciniVax BV based inGroningen, NETHERLANDS
Our first product candidate Vvax001, a therapeutic cancer vaccine against (pre)malignant cervical lesions, is currently in clinical development. We outsource process development and GMP manufacturing to experienced CMO’s, leading to a first clinical trial, which has been started in ...
Manufactured by:Vivesto AB based inSolna, SWEDEN
Docetaxel micellar is a product candidate in early clinical development and is a novel formulation that combines XR-17 with docetaxel - a well-established cytotoxin, currently administered intravenously and containing ethanol. In June 2020, Vivesto partnered with the Swiss Group for Clinical Cancer Research (SAKK) with the aim of conducting the ...
Manufactured by:Ascelia Pharma AB based inMalmö, SWEDEN
Oncoral is a novel daily irinotecan chemotherapy in development. Irinotecan chemotherapy has an established potent anti-tumor effect – even in difficult to treat cancers. Oncoral is a daily irinotecan tablet with the potential to offer better patient outcomes with improved safety following the daily dosing at home compared to intravenous high-dose infusions at the hospital. ...
Manufactured by:MorphoSys AG based inPlanegg, GERMANY
Tafasitamab (MOR208) is a humanized FC-modified CD19 targeting immunotherapy in clinical development for the treatment of B cell malignancies. CD19 is broadly expressed on the surface of B cells. It is therefore considered as a potential target for the treatment of B cell malignancies, such as non-Hodgkin’s lymphoma (NHL), including diffuse large B cell ...
Manufactured by:Aristotech Industries GmbH based inLuckenwalde, GERMANY
The Design of POROCUP is based on the principles of the well-known and long term clinically proven TITANIUM CUP developed in Switzerland in the 90´s. Applying a Titanium Forging Technology, a macro porosity surface is created, which favors the bone osteointegration without the necessity of additional ...