Show results for
Refine by
Assay Equipment Supplied In Belgium
16 equipment items found
Manufactured by:OraSure Technologies based inBethlehem, PENNSYLVANIA (USA)
As the leader in oral fluid specimen collection and testing, we proudly present our latest advancement in oral fluid collection with the Intercepti2 Oral Fluid Collection ...
Manufactured by:OraSure Technologies based inBethlehem, PENNSYLVANIA (USA)
The Intercept Oral Fluid Drug Test is an FDA-cleared laboratory-based oral fluid drug testing system that enables accurate testing for drugs of abuse including marijuana, cocaine, PCP, amphetamines and opiates. With a fast and easy-to-administer collection process, Intercept is ideal for workplace, criminal justice, drug treatment centers, and clinical setting screening programs, among ...
Manufactured by:OraSure Technologies based inBethlehem, PENNSYLVANIA (USA)
As the leader in oral fluid specimen collection and testing, we proudly present our latest advancement in oral fluid collection with the Intercept® i2he™ Oral Fluid Collection ...
Manufactured by:Biocartis NV based inMechelen, BELGIUM
The fully automated Idylla ctKRAS Mutation Test is minimally invasive, fast and easy to perform and can be used as complement to tissue testing to determine the RAS mutation status at diagnosis. IdyllaTM ctKRAS covers 21 KRAS mutations in exons 2,3 and 4 and showed an high concordance to NGS of 96% using plasma samples from 198 mCRC patients enrolled in the prospective multicenter RASANC ...
Manufactured by:Biocartis NV based inMechelen, BELGIUM
The fully automated Idylla BRAF Mutation Test covers BRAF V600E, E2, D, K, R and M mutation with a sensitivity of 1% of mutant in wild type background in FFPE samples with an excellent concordance to reference methods of ...
Manufactured by:Biocartis NV based inMechelen, BELGIUM
The fully automated Idylla EGFR Mutation Test covers 51 mutations in exons 18–21 in a single cartridge using only 1 FFPE tissue section from metastatic NSCLC showing a high concordance of >95% compared with reference ...
Manufactured by:Biocartis NV based inMechelen, BELGIUM
The fully automated Idylla KRAS Mutation Test covers 21 KRAS mutations in exons 2,3 and 4 showing an excellent concordance to reference methods of >95%. Comparison with various other KRAS detecting technologies revealed a superior performance of the IdyllaTM KRAS Mutation Test in terms of ease of use, turnaround time, and hands-on time while demonstrating superior levels of ...
Manufactured by:SYnAbs S.A. based inGosselies, BELGIUM
Final obtained rat mAbs make the difference between methylated and non-methylated lysine on histone DNA. An immuno-assay has been consequently developed under NuQ brand and is now ...
Manufactured by:OncoDNA S.A. based inGosselies, BELGIUM
It is used to monitor the progression of the tumor (burden of the disease) and to detect lack of response or resistance to treatment as soon as it appears. This assay is customised for each patient, as it analyses up to 15 variants previously identified in the tumour of the patient. In addition, it sequences a fixed panel of 40 genes associated with response/resistance to ...
Manufactured by:Avant Medical B.V. based inGeffen, NETHERLANDS
This state-of-the-art Lumi-Aggregometer measures platelet function on patient samples using electrical impedance in whole blood samples or optical density in plasma with the simultaneous measurement ATP Release by the luminescence method. The Model 700 Lumi-Aggregometer also has the capability of performing the Ristocetin Cofactor Assay for diagnosing patients with von ...
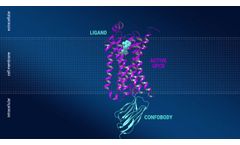
Manufactured by:Confo Therapeutics based inGhent/Zwijnaarde, BELGIUM
With its unique antibody-based technology, Confo Therapeutics aims to overcome the current limitations of GPCR drug discovery in order to fully realize the potential of GPCRs as drug ...
Manufactured by:SYnAbs S.A. based inGosselies, BELGIUM
When you perform intraperitoneal injection of hapten or highly homologous antigens in mouse species, most of the time your project is going to fail. Due to poor immunogenic properties and low molecular weight, your antigen is going to be eliminated in animal urine without generating any humoral immune response. That's why we've created SynAbs in the first place, based upon an exclusive asset: the ...
Manufactured by:ZenTech s.a. based inLiège, BELGIUM
The Targeted qPCR SMA uses the quantitative polymerase chain reaction to genotype the anterior spinal muscular atrophy (SMA) mutation in neonates on a dried blood sample. The test only identifies SMA homozygotes. This qualitative test is dedicated only to spinal muscular atrophy (SMA) newborn screening and is performed on a dried blood sample on 903® or 226 blotted paper. Parameter ; ...
Manufactured by:ZenTech s.a. based inLiège, BELGIUM
The Immunomat™ is intended for neonatal screening laboratories performing between 400 and 1000 daily determinations. With a maximum capacity of 7 microplates, it optimizes the rate of the laboratory by allowing the processing of different parameters in parallel. The handling of this robust instrument is easy and the software is intuitive to use. The Immunomat greatly facilitates and ...
Manufactured by:MDxHealth based inIrvine, CALIFORNIA (USA)
MDxHealth’s technology platform is called methylation-specific PCR (MSP), which is a proprietary DNA-based technology that functions on standard commercial PCR equipment. MSP is extremely powerful and accurate with the ability to detect a single cancer cell among 10,000 healthy cells in any type of bodily fluid or tissue. MDxHealth has patents and other intellectual property on the MSP ...
Manufactured by:MDxHealth based inIrvine, CALIFORNIA (USA)
ConfirmMDx Addresses Prostate Biopsy Sampling Error and the False-Negative Biopsy Dilemma. “Rule-in” high-risk men who have had a previous negative biopsy result, may be harboring undetected cancer (a false-negative biopsy result), and therefore may benefit from a repeat biopsy and appropriate treatment. “Rule-out” otherwise cancer-free men from undergoing unnecessary ...