- Home
- Equipment
- south korea
- automatic compact poct analyzer
Refine by
Automatic Compact Poct Analyzer Equipment Supplied In South Korea
14 equipment items found
Manufactured by:DxGen Corp. based inGunpo-si, SOUTH KOREA
Epithod AutoDx, a Fully-automatic analyzer, measures diabetes, infectious, and inflammation parameters quantitatively on an easy-to-use platform. It is suitable for physician offices, laboratories, and POC ...
Manufactured by:DxGen Corp. based inGunpo-si, SOUTH KOREA
Semi-Automatic Analyzer for Point-Of-Care Testing measuring HbA1c, Glycated Albumin, CRP, Hemoglobin, COVID-19 Antibody (IgM/IgG), and Antigen. ...
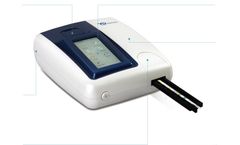
Manufactured by:YD-DIAGNOSTICS based inGyeonggi-do, SOUTH KOREA
Easy access to review test results (printout data selectable). Interface for the external barcode reader, PC and lab network system; Easy connect by 2 USB port. Reflection photometer :CCD color image sensor (image capture) and 3 LED for light source. Color and clarity reading available. 1.3" Touch Screen TFT LCD; Sofrware available in English, German, Spanish, Italian, Ploish, Chinese and ...
Manufactured by:SD Biosensor based inSuwon-si, SOUTH KOREA
STANDARD F Legionella Ag FIA test system (Analyzer + Test device) detects Legionella pneumophila serogroup 1, 3, 5, 6 and 8 antigens via urine sample. Without any further sample processing, STANDARD F Legionella Ag FIA performs highly sensitively, and the test is not affected by Rheumatoid ...
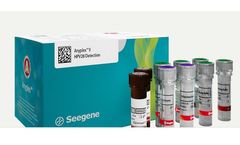
Manufactured by:Seegene Inc. based inSongpa-gu, SOUTH KOREA
Simultaneous detection and identification of 28 HPV types (19 high-risk and 9 ...
Manufactured by:Seegene Inc. based inSongpa-gu, SOUTH KOREA
Comprehensive assay for the detection and identification of 25 gastrointestinal pathogens using one-step real-time ...
Manufactured by:OPTOLANE Technologies Inc. based inSeongnam-si, SOUTH KOREA
LOAA Analyzer(model name : LOAA-M) is real-time PCR instrument which is an in vitro diagnostic (IVD) medical device used to amplify specific genes (DNA or RNA) designed for use with cartridges. PCR data is automatically analyzed by the dedicated software installed in the device and results are delivered to users through on-screen display or exported to external memory for further ...
Manufactured by:Seegene Inc. based inSongpa-gu, SOUTH KOREA
Comprehensive assay for the detection and identification of 26 pathogens using one-step real-time ...
Manufactured by:OPTOLANE Technologies Inc. based inSeongnam-si, SOUTH KOREA
LOAA Analyzer is Real-time PCR instrument which is an in vitro diagnostic (IVD) medical device used to amplify specific genes (DNA or RNA) designed for use with cartridges. PCR reaction data is automatically analyzed by the dedicated software installed in the device and results are delivered to users through on-screen display or export to external memory for further processing. ...
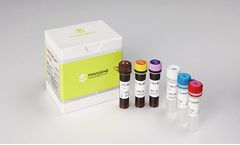
Manufactured by:Panagene Inc. based inDaejeon, SOUTH KOREA
According to the WHO report (2008), human papillomavirus (HPV) causes virtually 100% of cases of cervical cancers. There are more than 100 genotypes of HPV reported currently, and HPV consists of double helix circular DNA with ca. 7,900 bps. Compared to the traditional Pap smear test, HPV genotyping has several advantages; 1) high accuracy of determining existence of HPV viruses, 2) genotyping of ...
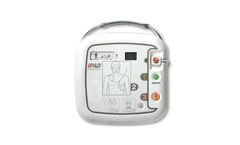
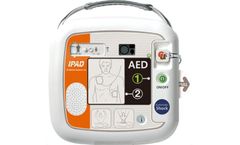
Manufactured by:CU Medical Systems, Inc. based inWonju-Si, SOUTH KOREA
The IPAD CU-SP1 is a semi-automated external defibrillator (AED). If connected to a patient, the IPAD CU-SP1 automatically acquires and analyzes the electrocardiogram (ECG) of the patient for the presence of Ventricular Fibrillation or Ventricular Tachycardia (also known as shockable rhythms). If a shockable rhythm is detected, the device automatically charges itself. Defibrillating shock is ...
Manufactured by:CU Medical Systems, Inc. based inWonju-Si, SOUTH KOREA
CU-SP1 AUTO is an easy-to-use Fully Automated External Defibrillator (AED) that is small, light, and portable, and uses a battery. The AED automatically reads the patient’s electrocardiogram (ECG) and determines if a cardiac arrest that requires defibrillation has occurred, so that both medical professionals and the general public can easily operate it. Cardiac arrest can occur anytime to ...
Manufactured by:YD-DIAGNOSTICS based inGyeonggi-do, SOUTH KOREA
Touch screen LCD: Menu driven 8.4″ large color touch LCD for convenient operation; Display the real view of the test strip image during operation. Cassette room: Double-sealed Super Strip Cassette for extended on-board stability; New Strip delivery System : Elevator system for strip loadeing. Interface: Various interfaces such ae 4 USB, RS232C, serial interface, 2 PS/2, parallel, Ethernet; ...
Manufactured by:Panagene Inc. based inDaejeon, SOUTH KOREA
PANAMutyper™ EML4-ALK Screening Kit is the qualitative diagnostic test kit which is able to detect 16 different EML4-ALK fusion genes of tissue from non-small cell lung cancer (NSCLC) patients using peptide nucleic acid (PNA) probes in a real-time PCR system. The PANAMutyper™ EML4-ALK Screening Kit is intended as an aid for screening EML4-ALK fusion-positive NSCLC patients who would ...