- Home
- Equipment
- north america
- bladder tumor
Show results for
Refine by
Bladder Tumor Equipment Supplied In North America
56 equipment items found
Manufactured by:Advin Urology based inAhmedabad, INDIA
TURP Sheath is an operative sheath through which transurethral electroreception of bladder tumors or the prostate gland can be performed. TURP Sheath provides continuous irrigation during TURP Procedures. It also provides 360 Degree movement of Working element which gives good resection of ...
Manufactured by:Veracyte, Inc. based inSouth San Francisco, CALIFORNIA (USA)
Revealing cancer molecular subtype to inform treatment decisions. Decipher Bladder is a genomic test that measures the molecular profile of bladder cancer using gene expression analysis from transurethral resected bladder tumor specimens. The test was developed for bladder cancer patients with high-grade ...
Manufactured by:INOVIQ Ltd based inNotting Hill, AUSTRALIA
INOVIQ has developed a proprietary immunocytochemistry (ICC) assay that detects hTERT, a component of telomerase, which is upregulated in most human epithelial ...
Manufactured by:Creative Peptides based inNew York, NEW YORK (USA)
Aplidine is a compound found in tunicates which shows promise in shrinking tumors in pancreatic, stomach, bladder, and prostate cancers. The specific marine organism is Aplidium albicans. Aplidine consists of peptide molecules. In addition to the cancers mentioned above it is also under consideration as a treatment for some types of ...
by:Antech Diagnostics based inFountain Valley, CALIFORNIA (USA)
Antech’s molecular diagnostics use and develop novel technologies that enable faster and more personalized diagnoses. With tests such as CADET® BRAF/PLUS and comprehensive PCR panels, we are the innovative leader in molecular research and testing. ...
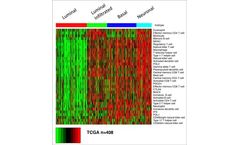
Manufactured by:GeneCentric Therapeutics, Inc. based inDurham, NORTH CAROLINA (USA)
Bladder cancer is one of the most common types of cancer which like lung and other cancers, is quite heterogeneous. Bladder cancer occurs more frequently in men than women, generally at an older age, and is more common among smokers than non-smokers. The most common type of bladder cancer begins within cells that line the inside of the bladder, urothelial cells – also called transitional ...
by:Imagin Medical based inAuburndale, MASSACHUSETTS (USA)
The conventional method of detecting bladder cancer uses white light during minimally invasive surgery to illuminate the bladder during a procedure called a cystoscopy. White light has been used for decades and is the standard for more than 90% of the market. In typical white light cystoscopy, a small camera is mounted at the end of the cystoscope. ...
Manufactured by:UroGen Pharma, Inc. based inPrinceton, NEW YORK (USA)
UroGen is developing UGN-102 (mitomycin) for intravesical solution as a primary nonsurgical alternative to repetitive TURBT. UGN-102 is an investigational formulation that utilizes our innovative technology, RTGel™ reverse-thermal hydrogel, for the treatment of low-grade NMIBC. Instilled via standard catheters, UGN-102 is designed to dwell for a period of several hours before being excreted ...
by:Imagin Medical based inAuburndale, MASSACHUSETTS (USA)
Introduced in 2010, blue light cystoscopy, uses blue-filtered white light to address the limitations of white light cystoscopy. This method involves administering a contrast agent into the bladder which is absorbed by the cancerous cells, causing them to fluoresce when exposed to blue light. Surgeons are then able to view images of the highlighted cancer cells and more ...
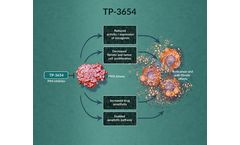
by:Sumitomo Pharma Oncology, Inc. based inCambridge, MASSACHUSETTS (USA)
TP-3654 is an oral investigational inhibitor of PIM kinases, which has potential antitumor and anti-fibrotic effects through multiple pathways, including induction of ...
Manufactured by:UroGen Pharma, Inc. based inPrinceton, NEW YORK (USA)
UGN-301 is UroGen’s in-licensed anti-CTLA-4 antibody (zalifrelimab). UGN-201 is our proprietary formulation of imiquimod, a toll-like receptor 7 (TLR 7) agonist. When combined, these medicines stimulate both the innate (UGN-201) and adaptive (UGN-301) immune responses, both of which are important for fully harnessing the power of the immune system to fight ...
Manufactured by:Nucleix Ltd. based inSan Diego, CALIFORNIA (USA)
Bladder cancer is the 5th most common cancer in the western world, and 70%-80% of the patients are diagnosed with non-muscle invasive disease. There are estimated 400,000 to 800,000 active bladder cancer patients in the United States, as well as 1 to 2 million in Europe, who undergo local resection of the tumor and then have 1-4 follow-up visits ...
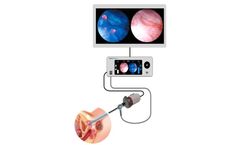
by:Imagin Medical based inAuburndale, MASSACHUSETTS (USA)
Imagin Medical’s i/Blue Imaging System addresses the limitations of both white and blue light cystoscopy while maintaining the advantages of both. By combining innovative optic and light sensor technology with the same FDA-approved imaging agent, surgeon’s will be able to view real-time, side-by-side white and blue light images on the same monitor, eliminating the need for surgeons to ...
by:Theralase Technologies Inc. based inToronto, ONTARIO (CANADA)
Theralase is laser focused on advancing the cutting-edge Anti-Cancer Therapy (“ACT”) towards commercialization, providing an alternative treatment methodology for Non-Muscle Invasive Bladder Cancer (“NMIBC”) patients globally. Theralase is dedicated to safely and effectively destroying cancer through the research, development and commercialization of light activated ...
Manufactured by:Pacific Edge Ltd based inDunedin,, NEW ZEALAND
Cxcolorectal is a prognostic gene signature for patients diagnosed with stage II or stage III colorectal cancer. The test predicts the aggressiveness of the tumour, allowing physicians to make the best decision regarding treatment for the ...
by:Tesis Biosciences based inScottsdale, ARIZONA (USA)
CGx Testing is a preventative gene test that looks for specific inherited changes (mutations) in a person’s genetic make-up. Harmful mutations may increase a persons’ chance, or risk, of developing a disease such as cancer. These conditions are considered hereditary. Appropriate genetic testing may be used to determine an individual’s risk and potential treatment options as ...
Manufactured by:BriaCell Therapeutics Corp. based inWest Vancouver, BRITISH COLUMBIA (CANADA)
The mechanism of action of Bria-IMT™/Bria-OTS™ is currently under investigation. We believe that Bria-IMT™/Bria-OTS™, activates the patient’s immune system to recognize tumor cells and destroy them. We hypothesize that Bria-IMT™/Bria-OTS™, exert their action by stimulating the antigen-presentation system (i.e. the system that presents antigen material on ...
Manufactured by:Protara Therapeutics, Inc. based inNew York, NEW YORK (USA)
TARA-002 is an investigational cell therapy in development for the treatment of non-muscle invasive bladder cancer (NMIBC) and lymphatic malformations (LMs). TARA-002 was developed from the same master cell bank of genetically distinct group A Streptococcus pyogenes as OK-432, a broad immunopotentiator marketed as Picibanil® in Japan and Taiwan by Chugai Pharmaceutical Co., ...
Manufactured by:miR Scientific, LLC based inNew York, NEW YORK (USA)
The science behind miR’s prostate and bladder cancer detection and classification technology that can help give healthcare providers, patients, and payors the greatest confidence in test results and care ...
Manufactured by:Cysview, Trademark of Photocure based inPrinceton, NEW JERSEY (USA)
Historically, bladder cancer patients are at high risk for disease recurrence and progression,1 which could be due in part to missed tumors and incomplete surgeries because not all cancerous tissue is easy to see under white light. Moving forward, with the availability of Blue Light Cystoscopy with Cysview, that trend may ...