Refine by
Cardiac Catheterization Equipment & Supplies
6 equipment items found
Manufactured by:Merit Medical Systems based inSouth Jordan, UTAH (USA)
The Prelude ACT (Activated Clotting Time) sheaths are part of Merit’s comprehensive family of vascular access products. These half-size sheaths allow easy blood draw through sheath side arm with or without catheters in place. The ACT sheaths accommodate a variety of catheters and other interventional devices to meet your clinical needs. ...
Manufactured by:Uscom Ltd based inSydney, AUSTRALIA
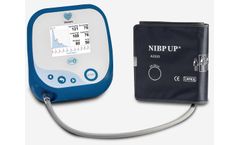
The Uscom BP+ is a Supra-systolic oscillometric central blood pressure (cBP) monitoring device which measures blood pressure and blood pressure waveforms at the heart, as well as in the arm; information only previously available using invasive cardiac ...
Manufactured by:Biocardia, Inc. based inSunnyvale, CALIFORNIA (USA)
The investigational CardiAMP™ Cell Therapy is designed to be a comprehensive biotherapeutic heart failure solution, incorporating: a proprietary molecular diagnostic to characterize the potency of a patient’s own bone marrow cells and determine if they are an optimal candidate for therapy. a point of care processing platform to prepare cells at the patient’s bedside. an ...
Manufactured by:EKU Elektronik GmbH
based inLeiningen, GERMANY
The maniNO blender for patient supply with a gas blend of oxygen (O2) and nitric oxide (NO) stands out due to easy usability and high NO-precision. It is suited for application in the manual ventilation and can thereby be used in transport as well as in MRI environment. The dosing range of maniNO can be adjusted from 0-40 ppm with an also steplessly adjustable total flow of 0-15 l/min. With a ...
Manufactured by:Transluminal Technologies, LLC based inSyracuse, NEW YORK (USA)
A first-of-its-kind magnesium alloy-based vascular closure device that offers a unique method of quickly achieving stable mechanical vascular closure following percutaneous catheterization for diagnostic or interventional procedures using 5-8 Fr procedural sheaths. The implant consists of a plug and footplate system that when presented to the arteriotomy, effects immediate, stable and reliable ...
Manufactured by:Abiomed based inDanvers, MASSACHUSETTS (USA)
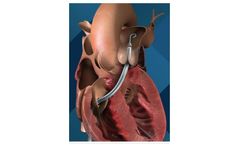
The Impella RP heart pump provides temporary, circulatory support for patients who develop right heart failure. It is the only FDA-approved heart pump indicated for patients experiencing acute right heart failure or decompensation following left ventricular assist device implantation, myocardial infraction, heart transplant or open-heart ...