Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Cellular Gene Therapy Equipment Supplied In Usa
201 equipment items found
Premium
Manufactured by:AB Sciex LLC based inFramingham, MASSACHUSETTS (USA)
Perform consistent and compliant biotherapeutics characterization faster than ever. With the PA 800 Plus system, you can confidently safeguard the success of your biologics. Run multiple characterizations from a single system that biopharma labs depend on, and produce qualitative and quantitative analyses, with speed and ...
Manufactured by:Xcell Biosciences Inc. based inSan Francisco, CALIFORNIA (USA)
Cell therapy manufacturing re-imagined. An automated, closed system for the production of autologous cell therapies. The KALI Cell Foundry is currently available for licensing ...
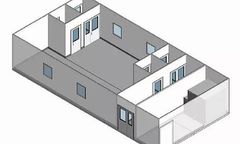
Manufactured by:G-CON Manufacturing, Inc. based inCollege Station, TEXAS (USA)
G-CON has developed a portfolio of six standardPOD Cleanrooms. These PODs are fully designed, allowing them to be mass-produced. Delivery time is as little as 3 months with the future goal of being able to maintain an inventory of ready to deliver PODs. Mass production of standardPODs will also lower costs. We believe cleanroom infrastructures have to become an off-the-shelf piece of ...
Manufactured by:Obsidian Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
Engineered with membrane-bound IL15, Eliminates toxic IL2 regimen, increasing patient accessibility to TIL therapy, Drives improved persistence, IL15 expression controlled by acetazolamide (ACZ), via cytoDRiVE® ...
Manufactured by:Ardigen based inKraków, POLAND
Accelerate discovery and improve safety of TCR therapies using Artificial Intelligence. Following the unique opportunity for curing patients provided by the development of cell therapies (e.g. TCR discovery), Ardigen has set on the path to advance the field with its Artificial Intelligence platform. Many challenges stand in the way of successful therapy discovery and development. Let us know how ...
Manufactured by:Gilead based inFoster City, CALIFORNIA (USA)
Sacituzumab govitecan-hziy 180 mg for injection. ...
Manufactured by:Orgenesis Inc. based inGermantown, MARYLAND (USA)
We leverage closed, automated processes that are validated for reliability and ...
Manufactured by:Polysciences, Inc. based inWarrington, PENNSYLVANIA (USA)
MAXgene GMP Transfection Reagent is a cGMP transfection reagent for the development and manufacturing of viral vectors for cell-and gene-based therapies. It is an ideal reagent in HEK293 and CHO systems for the manufacture of AAVs, LVs and recombinant proteins. MAXgene GMP capitalizes on the efficiency and scalability of Polysciences’ PEI MAX while adding the validation process and ...
Manufactured by:Charter Medical, LLC based inWinston-Salem, NORTH CAROLINA (USA)
Charter Medical’s Advect® Fluid Transfer Sets are designed to support the quality and manufacturing requirements of personalized care. The transfer sets are intended for sterile transfer of bone marrow mononuclear cells from one container to another. ...
by:Cytori Therapeutics Inc. based inSan Diego, CALIFORNIA (USA)
The Celution System is a medical technology developed by Cytori to automate and standardize the extraction and concentration of adult Adipose-Derived Regenerative Cells (ADRCs) in a clinical setting. The Celution System enables real-time access to autologous, clinical-grade ADRCs at the point-of-care facilitating cell therapy through the reimplantation or reinfusion of a patient’s own ...
Manufactured by:Altucell, Inc. based inNew York, NEW YORK (USA)
Encapsulated cell therapy is an emerging area of biopharmaceutical research that aims to unleash the therapeutic potential of cells to treat serious diseases without the need for immunosuppression. Altucell has developed patented, proprietary engineered cells that are combined with a patented encapsulation technology to create effective ...
Manufactured by:Pluristem Therapeutics Inc. based inHaifa, ISRAEL
Our convenient, automated thawing device, for use by clinicians at point of care, offers a reliable and simple thawing method that can safeguard the integrity of the supply chain. This is key to maintaining the potency and consistency of a cell product, because, unlike many traditional drugs, cell therapies must be thawed before being administered to a patient. However, the most commonly used ...
Manufactured by:Charter Medical, LLC based inWinston-Salem, NORTH CAROLINA (USA)
Freeze-Pak STS (FP-STS) Storage and Transport Solution Bio-Containers are designed for ultra-low temperature frozen storage and transport applications. FP-STS single-use containers offer robust performance to -80°C while the Freeze-Pak film itself is rated to temperatures as low as -196°C. FP-STS containers are suitable for long-term storage of cellular and non-cellular materials. The ...
Manufactured by:GeminiBio based inWest Sacramento, CALIFORNIA (USA)
GemCell Plus Xeno-Free Human Serum AB enables advanced therapy researchers and developers to streamline their workflow, allowing them to bring their end products to market quickly by providing human serum that is scalable, free of bovine components, uses therapeutic-grade human thrombin, and is rigorously ...
Manufactured by:Lineage Cell Therapeutics, Inc. based inCarlsbad, CALIFORNIA (USA)
The Lineage Platform begins with an infinitely reproducible cell line. Using our in-house manufacturing abilities and guided differentiation IP, we are able to direct pluripotent cells to become specialized cell types found in the human body, many of which have potential applications in transplant medicine. ...
Manufactured by:Gilead based inFoster City, CALIFORNIA (USA)
Brexucabtagene autoleucel . TECARTUS.TECARTUS®(brexucabtagene autoleucel)suspension for intravenous infusion. ...
by:Neurona Therapeutics based inSouth San Francisco, CALIFORNIA (USA)
A wholly-owned pipeline of novel cell therapy products. Neurona is developing allogeneic, off-the-shelf neural cell therapies for multiple chronic neurological disorders. The pipeline includes neuronal, glial, and gene-edited cell therapy candidates led by NRTX-1001, a first-in-class inhibitory neuron therapy for ...
by:Caribou Biosciences, Inc. based inBerkeley, CALIFORNIA (USA)
Our next-generation chRDNA genome-editing platform is the foundation for the development of differentiated allogeneic cell therapies. Our chRDNA technology enables multiple genome edits, including multiplex gene insertions, with a high degree of specificity and lower levels of off-target editing than first generation CRISPR-Cas9. ...
Manufactured by:Brooklyn ImmunoTherapeutics (BTX) based inSan Diego, CALIFORNIA (USA)
Cellular reprogramming refers to the process of transforming patient skin cells into pluripotent stem cells, called induced pluripotent stem cells (iPSC). Since 2012 Nobel Prize Winner Dr. Shinya Yamanaka's initial discovery of iPSC in 2006, iPSC availability has transformed the field of cell therapy. Brooklyn uses the fastest, highest-efficiency method for generating iPSC – a key ...
Manufactured by:Universal Cells Inc. based inSeattle, WASHINGTON (USA)
Pluripotent stem cells (PSCs) such as embryonic stem cells (ESCs) and induced PSCs (iPSCs) are undifferentiated stem cells that can differentiate into any cell type found in the body. Scientists around the world are using PSCs to produce more mature cell types with diverse therapeutic functions. These new cell therapies have the potential to change the lives of patients suffering from diseases of ...