Refine by
Cellular Therapy Equipment & Supplies
308 equipment items found
Manufactured by:Cellphire Therapeutics, Inc. based inRockville, MARYLAND (USA)
Cellphire Therapeutics is committed to transforming hemostasis management with innovative products that fill critical gaps in the treatment of bleeding patients. Our lead clinical-stage candidate, Thrombosomes, is an activated platelet-based hemostatic. The product is currently in a Phase 2 clinical trial in bleeding thrombocytopenic patients. Thrombosomes has also been given orphan drug ...
Manufactured by:NexImmune, Inc. based inGaithersburg, MARYLAND (USA)
Our AIM nanoparticle technology enables the development of immunotherapies that orchestrate specific T cell function and trigger a precise immune response against disease. An AIM™ Adoptive Cellular Therapy (ACT) product is manufactured using an AIM™ nanoparticle cocktail that contains multiple tumor-specific antigens and the AIM™ Enrichment and ...
Manufactured by:Adaptive Biotechnologies based inSeattle, WASHINGTON (USA)
We are currently leveraging our TCR discovery capabilities to enable commercialization of novel therapies by collaborators. In the future, we may explore expanding our end-to-end capabilities for the development of cellular therapies and vaccines. TruTCR is summarized in the figure ...
by:Dorian Therapeutics based inSan Carlos, CALIFORNIA (USA)
During aging, there is a decrease in the activity of stem cells, and therefore a reduced regenerative capacity. Additionally, senescent cells accumulate in the body and poison entire tissues. Dorian’s Senoblockers act on epigenetic regulators to reverse both processes, reactivating programs of youthfulness and regeneration. ...
Premium
Manufactured by:AB Sciex LLC based inFramingham, MASSACHUSETTS (USA)
Perform consistent and compliant biotherapeutics characterization faster than ever. With the PA 800 Plus system, you can confidently safeguard the success of your biologics. Run multiple characterizations from a single system that biopharma labs depend on, and produce qualitative and quantitative analyses, with speed and ...
Manufactured by:Mesoblast Ltd. based inMelbourne, AUSTRALIA
MPC-25-IC is Mesoblast’s Phase 2 product candidate for the treatment of acute myocardial infarction. This is the first clinical study to evaluate an allogeneic cellular therapy delivered by intracoronary infusion in patients who have suffered an acute myocardial ...
Manufactured by:Alcyomics Ltd. based inNewcastle Upon Tyne, UNITED KINGDOM
Our human tissue explant assay for the detection and characterisation of adverse immune reactions in vitro. Helping you characterise immune reactions to your novel chemicals, pharmaceuticals, biologicals and cellular ...
Manufactured by:Verax Biomedical, Incorporated based inMarlborough, MASSACHUSETTS (USA)
Stem cells, activated leukocytes and other cellular therapies are another area of obvious applicability for PGD technology as these cells are grown in media that also support bacterial proliferation. As with platelets and red blood cells, current techniques for the detection of contaminating bacteria before transplantation are limited to existing microbiological ...
Manufactured by:Albian Group based inBilbao, SPAIN
A physical barrier between a process that may require sterility and pressurisation, either positive or negative, from the outside environment, ensuring the necessary protection, reducing potential contamination and risks of cross-contamination and achieving the required air quality in the respective areas. ...
Manufactured by:Xcell Biosciences Inc. based inSan Francisco, CALIFORNIA (USA)
Cell therapy manufacturing re-imagined. An automated, closed system for the production of autologous cell therapies. The KALI Cell Foundry is currently available for licensing ...
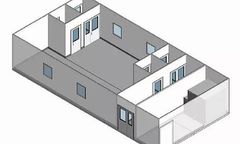
Manufactured by:G-CON Manufacturing, Inc. based inCollege Station, TEXAS (USA)
G-CON has developed a portfolio of six standardPOD Cleanrooms. These PODs are fully designed, allowing them to be mass-produced. Delivery time is as little as 3 months with the future goal of being able to maintain an inventory of ready to deliver PODs. Mass production of standardPODs will also lower costs. We believe cleanroom infrastructures have to become an off-the-shelf piece of ...
Manufactured by:Obsidian Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
Engineered with membrane-bound IL15, Eliminates toxic IL2 regimen, increasing patient accessibility to TIL therapy, Drives improved persistence, IL15 expression controlled by acetazolamide (ACZ), via cytoDRiVE® ...
Manufactured by:Ardigen based inKraków, POLAND
Accelerate discovery and improve safety of TCR therapies using Artificial Intelligence. Following the unique opportunity for curing patients provided by the development of cell therapies (e.g. TCR discovery), Ardigen has set on the path to advance the field with its Artificial Intelligence platform. Many challenges stand in the way of successful therapy discovery and development. Let us know how ...
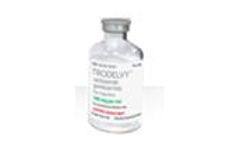
Manufactured by:Gilead based inFoster City, CALIFORNIA (USA)
Sacituzumab govitecan-hziy 180 mg for injection. ...
Manufactured by:Orgenesis Inc. based inGermantown, MARYLAND (USA)
We leverage closed, automated processes that are validated for reliability and ...
Manufactured by:Polysciences, Inc. based inWarrington, PENNSYLVANIA (USA)
MAXgene GMP Transfection Reagent is a cGMP transfection reagent for the development and manufacturing of viral vectors for cell-and gene-based therapies. It is an ideal reagent in HEK293 and CHO systems for the manufacture of AAVs, LVs and recombinant proteins. MAXgene GMP capitalizes on the efficiency and scalability of Polysciences’ PEI MAX while adding the validation process and ...
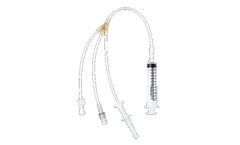
Manufactured by:Charter Medical, LLC based inWinston-Salem, NORTH CAROLINA (USA)
Charter Medical’s Advect® Fluid Transfer Sets are designed to support the quality and manufacturing requirements of personalized care. The transfer sets are intended for sterile transfer of bone marrow mononuclear cells from one container to another. ...
by:Cytori Therapeutics Inc. based inSan Diego, CALIFORNIA (USA)
The Celution System is a medical technology developed by Cytori to automate and standardize the extraction and concentration of adult Adipose-Derived Regenerative Cells (ADRCs) in a clinical setting. The Celution System enables real-time access to autologous, clinical-grade ADRCs at the point-of-care facilitating cell therapy through the reimplantation or reinfusion of a patient’s own ...
Manufactured by:Altucell, Inc. based inNew York, NEW YORK (USA)
Encapsulated cell therapy is an emerging area of biopharmaceutical research that aims to unleash the therapeutic potential of cells to treat serious diseases without the need for immunosuppression. Altucell has developed patented, proprietary engineered cells that are combined with a patented encapsulation technology to create effective ...
by:Chimeric Therapeutics based inCarlton South, AUSTRALIA
CHM 3301 (Undisclosed CAR NK) will be developed on the next generation CORE-NK platform leveraging a currently undisclosed Chimeric Antigen ...