Refine by
Class Ii Medical Device Equipment & Supplies
21 equipment items found
Manufactured by:Cala Health, Inc. based inBurlingame, CALIFORNIA (USA)
The Cala Trio device is an FDA-cleared Class II Medical Device for the external upper limb tremor stimulator* and is safe, effective, and individually calibrated to each patient's unique brain signal ...
Manufactured by:SprintRay Inc. based inLos Angeles, CALIFORNIA (USA)
High strength and resistance to wear make SprintRay Crown by BEGO® a go-to material for restorative 3D printing. And because SprintRay Crown by BEGO® is FDA 510(k) cleared and fulfills all the requirements for a Class II medical device, you can place with ...
Manufactured by:Inotec AMD based inCambridge, UNITED KINGDOM
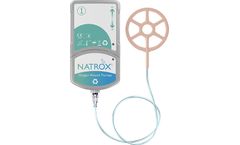
NATROX Oxygen Wound Therapy System is a class II medical device licensed under Medical Device Directive 93/42/EEC. The system consists of two main elements; the NATROX Oxygen Generator and the NATROX Oxygen Delivery System. ...
by:Click Therapeutics, Inc based inNew York, NEW YORK (USA)
CT-132 is under development as a clinically-validated digital program for ...
Manufactured by:Orlvision GmbH based inLahnau, GERMANY
The latest design in video endoscopy is the video-rhino-laryngoscope from orlvision. It was developed for endoscopy, image and video documentation especially for ENT physicians. International users confirm that the interaction of optics, illumination and camera electronics provides very meaningful ...
Manufactured by:Broncus Medical, Inc. based inSan Jose, CALIFORNIA (USA)
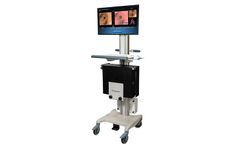
The Archimedes® Lite™ Virtual Bronchoscopic Navigation System, also known as LungPoint Plus® in Asia Pacific regions, is a simple, yet elegant virtual bronchoscopic navigation system that leverages the advanced navigation technology from Archimedes without fluoroscopy. Archimedes Lite is the only pulmonary navigation system that provides compatibility with any bronchoscope allowing ...
by:Click Therapeutics, Inc based inNew York, NEW YORK (USA)
Clickheart is under development as a clinically-validated digital program for acute coronary ...
Manufactured by:Chemence Inc based in, GEORGIA (US) (USA)
Our company is a world leader in cyanoacrylate technology research and has developed a wide range of high-performance grades of industrial cyanoacrylate adhesives based on ethyl, methyl, methoxyethyl, isopropyl, n-butyl and octyl monomers over the years. Servicing three main industries – medical, industrial and consumer – Chemence® continues to push the ...
by:Click Therapeutics, Inc based inNew York, NEW YORK (USA)
Clickadian is under development as a clinically-validated fully digital program for ...
by:Click Therapeutics, Inc based inNew York, NEW YORK (USA)
CT-161 is under development as a clinically-validated digital program for overactive ...
Manufactured by:Cirtec Medical based inBrooklyn Park, MINNESOTA (USA)
Long known for providing complete outsourcing solutions for Class II and III medical devices, Cirtec is pleased to announce we are now producing continuous draw Nitinol tubing. We understand that designing devices in a highly regulated industry can be challenging and our experienced and knowledgeable Nitinol team ...
Manufactured by:Emovi based inLaval, QUEBEC (CANADA)
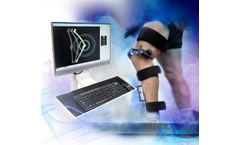
Optimize your athletes’ knee health. Build baselines for all athletes pre-season to help maintain knee health throughout the season. Support the athlete’s return to full performance with reduced pain and instability. Identify biomechanical markers that may be precursors to injury, thus helping to keep athletes healthier and on the ...
Manufactured by:Renishaw plc based inGloucestershire, UNITED KINGDOM
The neuromate system offers you the possibility to develop innovative and productive ways of ...
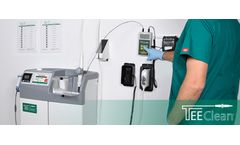
Manufactured by:CS Medical LLC based inCreedmoor, NORTH CAROLINA (USA)
TEEClean Automated TEE Probe Cleaner Disinfector is the first product of its kind to be cleared by the US FDA. Designed exclusively for transesophageal echocardiogram ultrasound probes (TEE), TEEClean provides both manufacturers recommended cleaning and high-level disinfection of soiled TEE probes in the same device. Current methods require manual cleaning to be completed so as to achieve proper ...
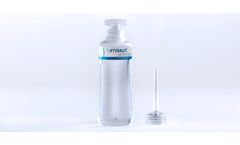
Manufactured by:Irrimax Corporation based inLawrenceville, GEORGIA (US) (USA)
Irrisept is jet lavage containing low concentration Chlorhexidine Gluconate (CHG) 0.05% in sterile water for irrigation. CHG acts as a preservative to help inhibit microbial growth in the ...
Manufactured by:AVACEN Medical based inCarlsbad, CALIFORNIA (USA)
*FDA- 510K Clearance -Indications for Use: The AVACEN is a heat therapy system indicated for the temporary relief of minor muscle and joint pain and stiffness; the temporary relief of joint pain associated with arthritis, muscle spasms, minor strains and sprains; muscular relaxation; and the temporary increase of local circulation where ...
Manufactured by:Acurable Limited based inLondon, UNITED KINGDOM
Clinically validated: AcuPebble SA100 automated sleep apnea test was validated in a powered clinical trial at the NHS Royal Free London hospital. Validated sleep study result equivalent to ambulatory gold-standard (multi-channel polygraphy followed by manual specialist interpretation). Validated result based on AHI and ODI for both 3% and 4% desaturation criteria with high accuracy (94% PPV, 98% ...
Manufactured by:Theralight, LLC based inLindon, UTAH (USA)
TheraLight 360 is a unique multi-wavelength LED system that combines 4 optimal therapeutic wavelengths to generate maximum results. 633nm Red Light, 810nm Near Infrared Light, 850nm Near Infrared Light, 940nm Near Infrared Light, Continuous Wave and Pulse Operating Modes, Wireless Control ...
Manufactured by:Revolutionary Science based inShafer, MINNESOTA (USA)
The FDA-approved, American Made Saniclave 102 is fashioned with a simple push-button operation. The thermally protective polymer skin eliminates burn risk. Low water and under and over-temperature alarms assure safety and effectiveness during operation. A corrosion-resistant, stainless steel chamber makes this steam sterilizer durable and easy to clean. The Saniclave 102 delivers exceptional ...
Manufactured by:NextPhase Medical Devices LLC. based inMansfield, MYANMAR
Looking for a turnkey, US-based, FDA-compliant EMD medical device manufacturer for your entire product life-cycle? We have a proven track record of reducing manufacturing costs by expediting development and production timelines while holding to the highest standards of quality. Our robust quality system has earned a strong reputation with some of the largest ...