- Home
- Equipment
- asia middle east
- clinic celiacshield trial
Show results for
Refine by
Locations
- Asia & Middle East
- Afghanistan
- Armenia
- Azerbaijan
- Bahrain
- Bangladesh
- Bhutan
- Brunei Darussalam
- Cambodia
- China
- East Timor
- Georgia
- Guam
- India
- Indonesia
- Iran
- Iraq
- Israel
- Japan
- Jordan
- Kazakhstan
- Kuwait
- Kyrgyzstan
- Laos
- Lebanon
- Malaysia
- Maldives
- Mongolia
- Myanmar
- Nepal
- North Korea
- Oman
- Pakistan
- Palestinian Territories
- Philippines
- Qatar
- Russia
- Saudi Arabia
- Singapore
- South Korea
- Sri Lanka
- Syria
- Taiwan
- Tajikistan
- Thailand
- Turkmenistan
- United Arab Emirates
- Uzbekistan
- Vietnam
- Yemen
Applications
- Flagship Coil for Major Depressive Disorder (MDD) Treatment
- Flagship Coil for Anxious Depression Treatment
- Flagship Coil for Obsessive-Compulsive Disorder (OCD) Treatment
- Flagship Coil for Smoking Addiction Treatment
- Flagship Coil for Bipolar Disorder Treatment
- Flagship Coil for Post Traumatic Stress Disorder Treatment Treatment
- Flagship Coil for Schizophrenia (Negative Symptoms) Treatment
- Flagship Coil for Alzheimer’s Disease Treatment
- Flagship Coil for Autism Disorder Treatment Technology
- Brain Computer Interface Technology for Emagine Stroke Recovery
Clinic Celiacshield Trial Equipment Supplied In Asia Middle East
131 equipment items found
Manufactured by:Troikaa Pharmaceuticals Ltd. based inAhmedabad, INDIA
Dynapar AQ is a novel Diclofenac injection manufactured by the Aquatech Process, which provides the full dose of 75 mg Diclofenac in just 1 ml. The Aquatech process provides the optimal concentration of Diclofenac in an aqueous solvent system, thereby, providing the full therapeutic dose in a smaller ...
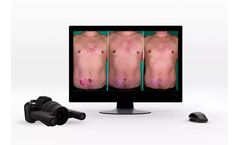
Manufactured by:Quantificare Inc. based inSuwanee, GEORGIA (US) (USA)
The 3D LifeViz® Series offers a range of high-quality 3D stereoscopic systems to consistently document any part of the body with unmatched accuracy and precision. All of our 3D systems are fully handheld and rely on a built-in ranging light system to ensure high-quality, reproducible images. Combined with our image analysis services, the 3D LifeViz® systems offer a powerful solution to ...
by:Vivozon Inc. based inYongin-si, SOUTH KOREA
A randomized, double-blind, placebo-controlled, single and repeated dose, escalation clinical trial to evaluate the safety, tolerability, and pharmacokinetics of VVZ-149 injection in healthy adult ...
Manufactured by:Quantificare Inc. based inSuwanee, GEORGIA (US) (USA)
The 2D DermaViz® is a fully handheld photography device equipped with fixed optics and a built-in ranging light system, ensuring high-quality, reproducible images. Designed for clinical staff use, this system is easy-to-use and delivers consistent results regardless of the extent of previous experience in photography. ...
by:Vivozon Inc. based inYongin-si, SOUTH KOREA
Single-dose, escalation clinical trial to evaluate the safety and pharmacokinetics of VVZ-149 injection in healthy elderly ...
Manufactured by:Troikaa Pharmaceuticals Ltd. based inAhmedabad, INDIA
Phlebotroy QPS is used in prevention and treatment of thrombophlebitis. Phlebotroy QPS is a new generation topical solution which ensures higher penetration of Heparin as compared to conventional ointment/gel. It is backed by multicentric clinical trials and is manufactured using the globally patented QPS technology. Each ml of Phlebotroy QPS contains 1000 lU/ml of Heparin ...
Manufactured by:Shanghai Henlius Biotech, Inc based inShanghai, CHINA
HLX14, recombinant anti-RANKL human monoclonal antibody injection, a denosumab biosimilar. It is potentially indicated for the treatment of postmenopausal women with OP at high risk for fracture. First subject has been doesd in the international multi-centre phase 3 clinical trial in China for the treatment of postmenopausal osteoporosis in women with high fracture ...
Manufactured by:Alnylam Pharmaceuticals, Inc. based inCambridge, MASSACHUSETTS (USA)
The first and only FDA-approved prescription medication for the treatment of primary hyperoxaluria type 1 (PH1). OXLUMO is a prescription medicine for the treatment of PH1 to lower oxalate in urine in children and ...
Manufactured by:Perspectum based inOxford, UNITED KINGDOM
Our digital pathology offering uses computer-generated, quantitative metrics to standardise biopsy analysis. We partner with ProPath to combine best-in class imaging with best-in-class pathology into a single integrated workflow for more objective, quantitative biopsy data. Our digital pathology service streamlines and improves decision-making in clinical trials with expert central review and ...
by:Vivozon Inc. based inYongin-si, SOUTH KOREA
A randomized, double-blind, parallel-group, placebo-controlled clinical trial to evaluate the analgesic efficacy and safety of VVZ-149 Injection in patients who have pain after laparoscopic and robotic-laparoscopic ...
by:Vivozon Inc. based inYongin-si, SOUTH KOREA
A multicenter, randomized, double-blind, parallel-group, placebo-controlled phase 3 clinical trial to evaluate the analgesic efficacy and safety of VVZ-149 injection in patients who have pain after laparoscopic ...
Manufactured by:Suvoda based inConshohocken, PENNSYLVANIA (USA)
Full visibility and automated control. Extend your clinical trial command and control center with Suvoda’s integrated ...
Manufactured by:Lifetech Scientific (Shenzhen) Co., Ltd. based inShenzhen, CHINA
Nitinol wire frame coated with Titanium Nitride (TiN). Decrease the dissolution of nickel ion efficiently, expected safe long-term biocompatibility. Promote the growth of endothelial tissue, lessen thrombus complication. Superior superelastic, effectively reduce atrioventricular block ...
Manufactured by:EMS Company based inNyon, SWITZERLAND
The Instrument PS (Perio Slim) is slim and smooth like a probe. It is gum-friendly, minimally invasive, maximally preventive, and preserves the epithelium thanks to its remarkably linear ...
Manufactured by:Shanghai Henlius Biotech, Inc based inShanghai, CHINA
HLX04-O, recombinant anti-VEGF humanised monoclonal antibody injection. It is indicated for wet age-related macular degeneration (wAMD). HLX04-O was granted Phase 3 clinical study approvals in Australia, the United States, Singapore and the EU countries such as Latvia, Hungary and Spanish. In April 2022, the first patient in Australia and Latvia was dosed in the global multicentre phase 3 ...
Manufactured by:Shanghai Henlius Biotech, Inc based inShanghai, CHINA
HLX07, recombinant anti-epidermal growth factor receptor (EGFR) humanised monoclonal antibody injection independently researched and developed as biobetter. It was granted IND approvals to be evaluated in clinical trials in China and the United States. It demonstrated favorable safety and tolerability profile in a prospective, open-label, dose-escalation Phase 1 clinical study (HLX07FIH, ...
by:Vivozon Inc. based inYongin-si, SOUTH KOREA
A randomized, double-blind, parallel-group, placebo-controlled clinical trial to evaluate the analgesic efficacy and safety of VVZ-149 Injection in patients who have pain after laparoscopic gastrectomy in early gastric cancer ...
by:Vivozon Inc. based inYongin-si, SOUTH KOREA
A randomized, double-blind, parallel group, placebo-controlled clinical trial to evaluate the analgesic efficacy and safety of VVZ-149 Injection in patients who have pain after artificial hip replacement ...
Manufactured by:Troikaa Pharmaceuticals Ltd. based inAhmedabad, INDIA
Dynapar QPS is the world's first Transdermal preparation of Diclofenac, which provides excellent pain relief after topical application, by enhancing the penetration of Diclofenac through the skin. Dynapar QPS is backed by proof of concept pharmacokinetic studies as well as clinical ...
Manufactured by:Shanghai Henlius Biotech, Inc based inShanghai, CHINA
HLX11, recombinant anti-HER2 domain II humanised monoclonal antibody injection, a pertuzumab biosimilar. It can be used in combination with trastuzumab and chemotherapy for adjuvant treatment and neoadjuvant treatment for patients with HER2-positive BC and HER2-positive mBC. The first subject has been dosed in multi-centre phase 3 clinical trial of HLX11 for the neoadjuvant therapy in patients ...